THERYA, 2024, Vol. 15(3):303-313 DOI:10.12933/therya-24-6132 ISSN 2007-3364
Distribution and karyotypic variation of Brazilian molossid bats (Chiroptera, Mammalia)
Lorena Silva de Souza1, Nathália Siqueira Veríssimo Louzada1, 2, Margaret Maria de Oliveira Corrêa1, and Leila Maria Pessôa1, 2*
1 Departamento de Zoologia, Instituto de Biologia, CCS, Universidade Federal do Rio de Janeiro. CCS Bloco A1-121 - Ilha do Fundão. Av. Carlos Chagas Filho, 373, CEP 21941-902, Brazil. Email: lorena.souza.2016@gmail.com (LSDS), louzada.tata@gmail.com (NSVL), margaret.correa2016@gmail.com (MMDOC), pessoa@acd.ufrj.br (LMP).
2 Postgraduate Program in Biodiversity and Evolutionary Biology, Institute of Biology, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
*Correspondig author: https://orcid.org/0000-0003-2468-5190.
Recent biogeographic studies have shown that geographically distant populations of different animal groups, including bats, can present genetic differentiation. Given this, the objective here was to study the composition of Molossidae species that occur in Brazil and investigate whether these species present karyotypic differences between populations from different biomes. A bibliographical survey was carried out, and the karyotypes found for each species were analyzed and compared in relation to the diploid number of chromosomes, the fundamental number of arms of the autosomal chromosomes, the centromeric position, and the presence of other structural chromosomal variations. The results showed that of the 32 species of Molossidae recorded for Brazil, 14 have their karyotypes described. Of these, only nine refer to specimens collected in Brazil. For Molossus molossus, karyotypic variations were observed between the Amazon and the Atlantic Forest, and within the Amazon, in regions separated by the Amazon River. Molossops temminckii showed variations among populations in the state of Minas Gerais. Eumops perotis and Cynomops planirostris also showed differentiation between populations from the Amazon and the Atlantic Forest. Molossus rufus showed variation between populations from the Atlantic Forest and Caatinga. The variations observed were structural in autosomal and sexual chromosomes between different populations. The species Cynomops abrasus, Eumops glaucinus, and Nyctinomops laticaudatus have studies only for the Atlantic Forest region, while Eumops hansae has karyotypic studies only for the Amazon region. This study showed the existence of karyotypic variation between different populations of five species of molossids. Furthermore, it highlighted the need for investment in knowledge of family karyology, which is scarce, with the aim of better understanding aspects of karyotypic evolution in this group.
Estudios biogeográficos recientes han demostrado que poblaciones geográficamente distantes de diferentes grupos de animales, incluidos los murciélagos, pueden presentar diferenciación genética. Ante esto, el objetivo fue estudiar la composición de las especies de Molossidae presentes en Brasil e investigar si estas especies presentan diferencias cariotípicas entre poblaciones de diferentes biomas. Se realizó un levantamiento bibliográfico y los cariotipos encontrados para cada especie fueron analizados y comparados en relación con el número diploide de cromosomas, el número fundamental de brazos de los cromosomas autosómicos, la posición centromérica y la presencia de otras variaciones cromosómicas estructurales. Los resultados mostraron que de las 32 especies de Molossidae registradas para Brasil, 14 tienen el cariotipo descrito. De ellos, sólo nueve se refieren a ejemplares recolectados en Brasil. Para Molossus molossus, se observaron variaciones cariotípicas entre la Amazonía y la Mata Atlántica, y dentro de la Amazonia, en regiones separadas por el río Amazonas. Molossops temminckii mostró variaciones entre poblaciones en el estado de Minas Gerais. Eumops perotis y Cynomops planirostris también mostraron diferenciación entre poblaciones de la Amazonía y la Mata Atlántica. Molossus rufus mostró variación entre poblaciones de la Mata Atlántica y Caatinga. Las variaciones observadas fueron estructurales en los cromosomas autosómicos y sexuales entre diferentes poblaciones. Las especies Cynomops abrasus, Eumops glaucinus y Nyctinomops laticaudatus tienen estudios sólo para la región de la Mata Atlántica, mientras que Eumops hansae tiene estudios cariotípicos sólo para la región amazónica. Este estudio mostró la existencia de variación cariotípica entre diferentes poblaciones de cinco especies de molósidos. Además, destacó la necesidad de invertir en el conocimiento de la cariología familiar, que es escaso, con el objetivo de comprender mejores aspectos de la evolución cariotípica en este grupo.
Keywords: Brazil; karyological variation; Free-tailed bats; Molossidae; Phytophysionomy.
© 2024 Asociación Mexicana de Mastozoología, www.mastozoologiamexicana.org
Introduction
Molossids are known as free-tailed bats, morphologically characterized by a tail that extends about one-third beyond the outer edge of the uropatagium and the presence of a “hairbrush” on the outer toes (Gregorin and Cirranello 2016). This family has fast and long-lasting flight, adapted to open areas, which is reflected in their aerodynamic design of head, ears, and wings, and the quadrupedal habit, rare in Chiroptera (Vaughan 1966). The Molossidae family currently includes 23 genera and 132 described species, with a circumtropical distribution (Gregorin and Cirranello 2016; Simmons and Cirranello 2023; see Wilson and Mittermeier 2019). In Brazil, eight genera and 32 species are registered, widely distributed throughout the national territory (Garbino et al.20224).
Currently, many studies have been carried out on the processes that resulted in the current patterns of distribution and differentiation of organisms. These works have shown that climate fluctuations promoted cycles of expansion and contraction of different vegetation formations in Brazil (Costa 2003; Werneck et al. 2012; Batalha-Filho et al. 2013). These events would have influenced the dispersal, genetic differentiation of populations, and speciation of different organisms, such as birds (Beven et al. 1984; Batalha-Filho et al. 2013), reptiles (Werneck et al. 2012), rodents (Costa 2003), and bats (Martins et al. 2009; Pavan et al. 2011; Silva et al. 2023).
Dealing with Chiroptera in more detail, a study carried out by Martins et al. (2009)) on Desmodus rotundus (Phyllostomidae) indicated through molecular analyses that there is a genetic structuring of species populations coinciding with the division of biomes in South America. Pavan et al., (2011) showed that Carollia perspicillata and C. brevicauda also exhibited genetic structuring of their populations throughout their distribution, coinciding with phytogeographic variation. A study carried out by Loureiro et al. (2020a) showed that some species of the genus Molossus presented structuring between populations distributed both on the continent and on islands suggesting a certain degree of genetic differentiation between populations of different species of the genus north and south of the Amazon River. Therefore, it is possible that other species in the family have been influenced by geoclimatic processes, such as the separation between humid forests, affecting gene flow and leading to the accumulation of distinct characteristics between populations.
Among the several ways of accessing the evolutionary differences between different taxa is cytogenetics, a field of study in biology that, based on the use of different techniques, allows the observation of numerical and structural characteristics of the chromosomes of distinct organisms (Varella-Garcia and Taddei 1989). Cytogenetics is, therefore, a method of studying karyotypic diversity and the variations that exist between different individuals, populations, species, and biological groups.
This field of research has been aiding in the process of identifying species, especially in groups where there may be taxonomic controversies, such as Rodentia (Bonvicino and Weksler 1998; Christoff et al. 2000) and Chiroptera (Eick et al. 2007; Ao et al. 2006; Moratelli et al. 2007; de Lemos Pinto et al. 2012). Furthermore, this tool can be very important for understanding the biogeography of some groups, such as the African murids studied by Granjon and Dobigny (2003), the Neotropical cichlids studied by Thompson (1979), and the species Rhinophylla fischerae from Phyllostomidae, which may represent more than one species, according to geographic variation in the karyotype (Gomes et al. 2010). For molossids, however, studies have generally focused on describing the karyotypes of the species without aiming to cytogenetically compare species collected in different regions or biomes of Brazil. The studies by Morielle-Versute et al. (1996) and Corrêa (2016), for example, described the karyotype of the species Cynomops planirostris and Molossus molossus for the Atlantic Forest region and the Amazon, respectively. However, no studies regarding possible karyotypic variations between populations of these species have been conducted. Seeking this information is of great value from a conservation perspective, which aims to protect the diversity and uniqueness of species and their populations (Palacios-Mosquera et al. 2020), as well as from an evolutionary perspective, which aims to understand the biological processes that led to current diversity (Santos et al. 2019).
Given this, the central objective of the present study was to conduct a survey of Molossidae species that occur in Brazil and investigate whether they exhibit structural and/or numerical karyotypic differences between their populations. In more detail, the objectives were: (i) to map the distribution of molossid species in Brazil, identifying their distribution across different biomes; (ii) to carry out a karyotypic survey of these species; and (iii) to comparatively analyze the karyotypes obtained from each species, aiming to identify karyotypic variations between populations from different biomes.
Materials and methods
The survey of the karyotypic descriptions of the species and their occurrence records was carried out by consulting the online databases Google Scholar, SCIELO (The Scientific Electronic Library Online), BHL (Biodiversity Heritage Library), GBIF (Global Biodiversity Information Facility), and ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade). The nomenclature of bat species follows Garbino et al. (2022).
A compilation of occurrence records obtained from bibliographic research was carried out to understand the distribution of species across different biomes. The locations of occurrence records for each species were plotted on distribution maps, created using QGIS software. Additionally, the locations of the karyotypes described in the literature were also indicated on maps.
The karyotypes obtained through bibliographic research were reorganized according to the morphology and position of the centromeres, following Levan et al. (1964). The karyological data for each species were analyzed in relation to the diploid number of chromosomes (2n), the fundamental number of autosomal chromosome arms (FN), the centromeric position, and the presence of other structural chromosomal variations. Subsequently, comparisons were made between the different karyotypes described in the literature for each species.
Results
Distribution of species by Biome. Currently, there are records in Brazil for eight genera and 32 species of molossids (Garbino et al. 20234), which are widely distributed across Brazilian biomes. The species distribution data in the surveyed biomes are organized and summarized in Table 1.
Based on the bibliographic survey of distribution data carried out in this study, it was observed that three of the molossid species recorded in Brazil (Cynomops mastivus, Cynomops milleri and Eumops trumbulli) occur only in the Amazon region. Two species (Eumops chimaera and Molossus fluminensis) have records for the Atlantic Forest in Brazil, both with a probable distribution in the Cerrado and Pantanal, since they are also recorded in Bolivia (Taylor et al. 2019). Molossops neglectus occurs only in the Amazon and the Atlantic Forest. Cynomops greenhalli is found in the Amazon and Caatinga, while Molossus currentium has records in the Amazon and Pantanal. Eumops bonariensis is concentrated in the Atlantic Forest, Cerrado, and Pampas; Eumops patagonicus is found in the Atlantic Forest, Pantanal, and Pampas; and Nyctinomops aurispinosus occurs in the Atlantic Forest, Caatinga, and Cerrado. Molossus pretiosus is recorded only in the Cerrado, Pantanal, and NW Amazon. The remaining 20 species have broader occurrences in Brazilian biomes (Taylor et al. 2019).
Karyotypic variation in Brazilian species. Of the 32 species of Molossidae recorded for Brazil, 14 have published karyotypic descriptions, with only nine referring to specimens collected in Brazil (Table 2). Cynomops planirostris, Eumops perotis, and Molossus molossus have karyotypic studies for both the Amazon and Atlantic Forest regions. Molossus rufus has karyotype studies in the Atlantic Forest, Cerrado, and Caatinga regions. Cynomops abrasus, Eumops glaucinus, Molossops temminckii, and Nyctinomops laticaudatus have studies only for the Atlantic Forest region, while Eumops hansae has a karyotypic study only for the Amazon region (Table 2).
The next section details the distribution of species with available karyotypic studies available in the literature and describes the karyotypic studies identified in the bibliographic survey.
Distribution and description of the karyotypic variation of the species
Molossus E. Geoffroy, 1805
Molossus molossus (Pallas, 1766)
Type locality: Martinique, Lesser Antilles.
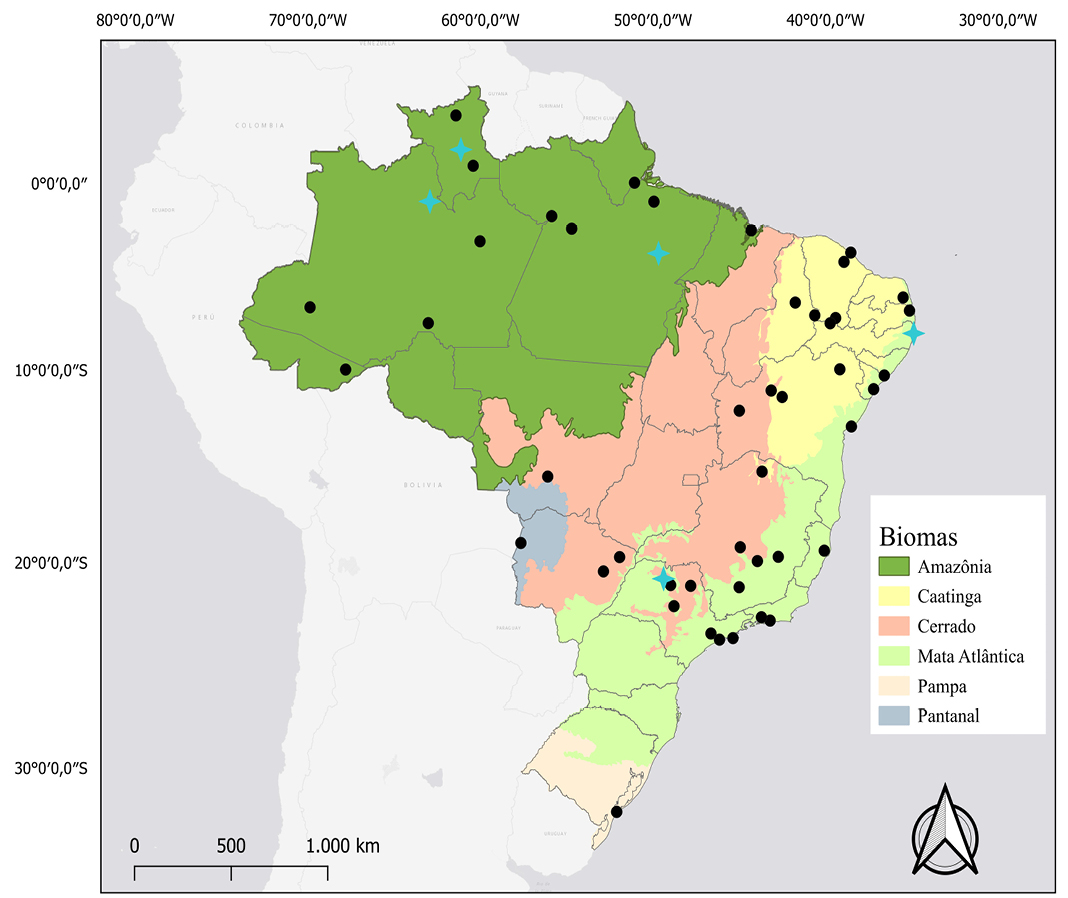
Distribution: The distribution of Molossus molossus is extensive, covering southern North America, Central America, and South America. Records indicate its occurrence in all Brazilian biomes (Peracchi et al. 2011; Barros et al. 2014; Loureiro et al. 2018; Figure 1).
Karyology: The karyotypes described for Molossus molossus by Lopes (1978) for eastern Pernambuco and by Cristoff and Freitas (1987) for Rio Grande do Sul, according to Varella-Garcia et al. (1989), presented 2n = 48 and FN = 56. However, other karyotypes described for regions in the Amazon (Corrêa and Bonvicino 2016) and Atlantic Forest (Morielle-Versute et al. 1996) have 2n = 48 and FN = 64. Karyotypic studies for Molossus molossus in Brazil generally describe the species' autosomal chromosomes as consisting of one pair of very large submetacentrics, three pairs of medium submetacentrics, five pairs of medium to small subtelocentrics and 14 pairs of medium to small acrocentrics (Morielle-Versute et al. 1996; Faria 2003; Brandão 2015). Leite-Silva et al. (2003) describe a large submetacentric pair, eight pairs of medium-sized two-armed chromosomes, and 14 medium-to-small-sized acrocentric pairs. Corrêa (2016) describes a pair of large metacentric chromosomes, eight pairs of metacentric or submetacentric chromosomes, ranging from large to medium, and 14 pairs of acrocentric chromosomes, ranging from large to small.
Regarding sex chromosomes, Morielle-Versute et al. (1996) and Leite-Silva et al. (2003) describe the X chromosome as a medium-sized submetacentric and the Y chromosome as a small subtelocentric. Faria (2003) described the X chromosome as a medium-sized submetacentric and the Y chromosome as a small acrocentric. Corrêa and Bonvicino (2016) described the X chromosome as a large metacentric and the Y chromosome as a small acrocentric.
Molossus rufus E. Geoffroy, 1805
Type locality: Caiene, French Guiana.
Distribution: Molossus rufus is widely distributed in South America. However, Loureiro et al. (2020b) recently revalidated Molossus fluminensis as a distinct species, with type locality at Rio de Janeiro, and previously considered a junior synonym of Molossus rufus. Loureiro et al. (2020b) describe the distribution of M. fluminensis as being in southeastern Brazil, Bolivia, Paraguay, and Argentina, and the distribution of M. rufus as being in central and northern Brazil, Bolivia, Ecuador, French Guiana, Guyana, Peru, Suriname, Trinidad and Tobago, and Venezuela. In Brazil, M. rufus is recorded in the Amazon, Caatinga, Cerrado, Atlantic Forest. M. fluminensis is recorded in Atlantic Forest, Cerrado, Pantanal, and Pampa (Souza et al. 2016; Loureiro et al. 2018; Loureiro et al. 2020b; Figure 2).
Karyology: In studies carried out by Morielle-Versute et al. (1996), Faria (2003), and Leite-Silva et al. (2003) with specimens collected in the Atlantic Forest, the species Molossus rufus presented 2n = 48 and FN = 64. In the study by Leal (2012) for the Caatinga, 2n = 48 and FN = 62 was found. Leal (2012) also mentions that Dantas (2004) and Sousa (2007) also found 2n = 48 and FN = 62 in their studies with specimens collected in Pará and Piauí, respectively.
The karyotypic studies of Molossus rufus conducted by Morielle-Versute et al. (1996) and Faria (2003) describe the species' autosomal chromosomes as consisting of one pair of large submetacentrics, three pairs of medium submetacentrics, five pairs of medium to small subtelocentrics, and 14 pairs of medium to small acrocentrics. The study by Leite-Silva et al. (2003) describes the karyotype as having a large submetacentric pair, eight pairs of medium-sized two-armed chromosomes, and 14 medium- to small-sized acrocentric pairs. The study by Leal (2012) reports eight pairs of metacentric and submetacentric chromosomes, one of which is large and the others medium in size, along with 15 acrocentric pairs that range from medium to small. Sex chromosomes were characterized by Morielle-Versute et al. (1996) and Leite-Silva et al. (2003) as having an X chromosome that is medium-sized and submetacentric, and a Y chromosome that is small and subtelocentric. According to Faria (2003) and Leal (2012), the X chromosome is medium-sized and submetacentric, while the Y chromosome is small and acrocentric.
Molossops Peters, 1866
Molossops temminckii (Burmeister, 1854)
Type locality: Lagoa Santa, Minas Gerais, Brazil.
Distribution: Molossops temminckii occurs in South America, with records in Argentina, Bolivia, Brazil, Colombia, Ecuador, Guyana, Paraguay, Peru, Uruguay, and Venezuela (Eger 2008). In Brazil, it has been recorded in the Amazon, Caatinga, Cerrado, Atlantic Forest, and Pantanal (Nunes et al. 2013; Figure 3).
Karyology: The karyotypic studies of Molossops temminckii conducted by Morielle-Versute et al. (1996) and by Santos (2013) observed 2n = 48 and FN = 68. The karyotypic morphology of autosomal chromosomes was described by Morielle-Versute et al. (1996) as one large submetacentric pair, two medium-sized submetacentric pairs, eight subtelocentric pairs, and 12 medium-to-small acrocentric pairs. Santos (2013) described three metacentric, four submetacentric, five subtelocentric, and 10 acrocentric pairs. It is noted that there may be an error in chromosome counting in Santos (2013), with possibly 11 pairs of acrocentric chromosomes instead of 10. Morielle-Versute et al. (1996) described the sex chromosomes with the X as a medium-sized subtelocentric and the Y as a small subtelocentric. Santos (2013) only analyzed the karyotype of a female, thus describing the X chromosome as subtelocentric.
Eumops Miller, 1906
Eumops perotis (Schinz, 1821)
Type locality: Campos dos Goytacazes, Rio de Janeiro, Brazil.
Distribution: According to Eger (1977), Eumops perotis has two disjoint populations: one in North America, identified as Eumops perotis californicus, and another in South America, recognized as Eumops perotis perotis. In Brazil, it has been recorded in the Amazon, Caatinga, Cerrado, Atlantic Forest, and Pantanal (Torres et al. 2020; Figure 4).
Karyology: According to Varella-Garcia (1989), Toledo (1973) describes 2n = 48 and FN = 54. Studies by Corrêa (2016) for the Amazon and by Morielle-Versute et al. (1996) and Okumura Finato et al. (2000) for the Atlantic Forest presented 2n = 48 and FN = 58. Morielle-Versute et al. (1996) described autosomal chromosomes as one pair of large submetacentric chromosomes, three pairs of medium submetacentric chromosomes, two pairs of subtelocentric chromosomes, and 17 pairs of acrocentric chromosomes, ranging from medium to small. Corrêa (2016) describes a pair of large metacentric chromosomes, five pairs of medium metacentric or submetacentric chromosomes, and 17 pairs of acrocentric chromosomes, ranging from medium to small. In the study by Morielle-Versute et al. (1996), the X chromosome is described as medium submetacentric and the Y chromosome as small acrocentric. In Corrêa (2016), the X chromosome may have been mistakenly described as a medium metacentric, as only females were analyzed, so it is not possible to rule out that it is the same.
Cynomops Thomas, 1920
Cynomops planirostris (Peters, 1866)
Type locality: Caiene, French Guiana.
Distribution: Cynomops planirostris is known from Venezuela, Guianas, Suriname, eastern Colombia, Peru, Bolivia, Paraguay, Argentina, and Brazil (Eger 2008). In Brazil, it has been recorded in all biomes except the Pampa (Santos et al. 2015; Mendes et al. 2020; Figure 5).
Karyology: The karyotype study by Santos (2013), carried out with specimens collected in Itinga (MG) in the central region of the Atlantic Forest, showed 2n = 34 and FN = 64. The autosomal chromosomes were described as 13 meta- or submetacentric pairs, two subtelocentric pairs, and one acrocentric pair. The studies by Leite-Silva et al. (2003), for the northern region of the Atlantic Forest and Corrêa (2016) for the Amazon presented 2n = 34 and FN = 60, with autosomal chromosomes described as 14 pairs of metacentric or submetacentric chromosomes, ranging from large to small, and two pairs of acrocentric chromosomes. The X chromosome in was described as a large metacentric, in Leite-Silva et al. (2003) as a medium submetacentric, and in Corrêa (2016) as a medium metacentric. The Y chromosome in was described as a small metacentric, while in Leite-Silva et al. (2003) and Corrêa (2016) it was described as a small acrocentric.
Discussion
Status of cytogenetic knowledge of molossids in Brazil. Our results showed that although the Molossidae family has 32 species in Brazil, there is limited knowledge about their karyology. Through our bibliographical research, we identified a total of 25 karyotypic studies that included molossid species occurring in Brazil. Of these, 11 studies were carried out with specimens collected within the country (Table 2). It is worth noting that, in general, the studies found were focused on describing the karyotypes of the species but did not aim to investigate karyotypic variations between populations from different phytogeographic regions in Brazil, as is the focus of the present study.
As highlighted in our results, only 14 molossid species have a karyotypic description. When we restrict this number to karyotypes from specimens collected in Brazil, only nine species have been studied (Table 2). Furthermore, even the species that have been studied do not have karyotype descriptions for all the different regions in which they occur in the country. For example, Cynomops abrasus, Eumops glaucinus, Molossops temminckii, and Nyctinomops laticaudatus have a wide distribution but only have karyotypic studies within the Atlantic Forest (tables 1 and 2).
The work carried out by Morielle-Versute et al. (1996) was one of the first studies on Brazilian molossids, presenting the karyotypes of seven species. This study was of great importance for understanding the karyotypic variation within the family in Brazil. Since then, other studies have been conducted; however, the karyology of populations of 23 species remains unknown and needs to be investigated.
According to Sotero-Caio et al. (2017), approximately 50 % of molossid bat species have been studied using conventional staining (Giemsa) worldwide, 11 species have been analyzed with G-banding, and four species have been analyzed with the Zoo-FISH technique. Of the species studied, 41 had 2n = 48, with the FN varying between 54 and 66. In nine species, the diploid number varied widely, ranging from 34 to 52 (Tables 2). The authors highlight the challenges in determining the number of chromosomal arms in Molossidae, as many species have numerous subtelocentric chromosomes. These difficulties are also related to the level of chromosome condensation and the quality of chromosome preparation. Nevertheless, accurate identification of chromosomal arms is considered crucial for understanding the karyotypic evolution of the family.
Due to the limited variation in diploid and fundamental numbers found in the literature, the most accepted hypothesis is that karyotypic conservatism characterizes the evolution of Molossidae. This conservatism is primarily attributed to pericentric inversions, Robertsonian rearrangements, and translocations (Morielle-Versute et al. 1996; Sotero-Caio et al. 2017). According to Sotero-Caio et al. (2017), although chromosomal evolution in Molossidae is generally conservative, intrageneric variations were identified within Cynomops and between the genera Cynomops and Molossops, as reported by Leite-Silva et al. (2003). Intraspecific variations were also observed in Eumops glaucinus, as documented by Warner et al. (1974), with differences found between specimens from Colombia (2n = 40, NF = 64) and those from Mexico and Costa Rica (2n = 38, NF = 64). These variations may have been influenced by geographic factors.
Chromosomal variations in Brazilian molossids. In this section, we will individually discuss the variations found between the karyotypic studies carried out for each species.
Molossus molossus. According to Morielle-Versute et al. (1996), the differences in FN observed between the studies by Lopes (1978) and Cristoff and Freitas (1987) compared to others are attributed to the observation of less condensed metaphases obtained from fibroblast cultures. Although the most recent studies do not show numerical chromosomal variations, they do reveal structural differences in autosomal and sexual chromosomes.
In general, the karyotypic descriptions by Morielle-Versute et al. (1996), Faria (2003), and Brandão (2015) are more like each other compared to the description by Corrêa (2016). The latter study not only showed greater differences in autosomal chromosomes but also in the X chromosome. This differentiation aligns with findings by Loureiro et al. (2020a), which indicated genetic differentiation between molossid populations separated by the Amazon River. Corrêa's study was carried out with specimens collected in Barcelos (AM) and Caracaraí (RR), north of the Amazon River, while the other studies focused on populations south of the river. However, further cytogenetic and molecular studies are needed to explore this hypothesis, including research across other South American biomes.
Regarding the description of the Y chromosome, no direct relationship can be identified concerning population differentiation between the Amazon and Atlantic Forest biomes or within the Atlantic Forest biome itself. Both the study by Morielle-Versute et al. (1996), which described the Y chromosome as subtelocentric, and the study by Faria (2003), which described it as acrocentric, were conducted with specimens collected in the Atlantic Forest region of São Paulo. Additionally, the study by Leite-Silva et al. (2003), which also identified the Y chromosome as subtelocentric, was based on specimens from Pernambuco. Therefore, variations in the Y chromosome of Molossus molossus require further investigation.
Molossus rufus. Molossus rufus exhibited both numerical and structural chromosomal variations between studies conducted in the Atlantic Forest and those from the Amazon, Caatinga, and Cerrado. Studies from the southern Atlantic Forest provided consistent descriptions for autosomal chromosomes. However, the northern Atlantic Forest study could not adequately identify the centromeric positions of these chromosomes. Research in the Caatinga and Cerrado showed identical 2n and FN values but differed from other biomes in the centromeric positions of chromosomes. Regarding sex chromosomes, no variation was observed for the X chromosome. The Y chromosome did show variation among studies, but this variation does not appear to be related to the different biomes.
The karyotypic variation found among specimens collected in the Atlantic Forest and other biomes may be interspecific. Considering the distribution proposed by Loureiro et al. (2020b), in which M. fluminensis occurs in southeastern Brazil, Bolivia, Paraguay, and Argentina, and M. rufus in the central and northern regions of Brazil, Bolivia, Ecuador, French Guiana, Guyana, Peru, Suriname, Trinidad and Tobago, and Venezuela, it is possible that the karyotyped specimens from the Atlantic Forest actually correspond to M. fluminensis. However, there are questions regarding the actual identification of the specimens in relation to the karyotypic samples from the Cerrado, Caatinga, and Atlantic Forest in the northeast region, since the samples used by Loureiro et al. (2020b) that separate the two species molecularly are from the Guianas and southeastern Brazil. According to the distribution proposed by the author, the FN of M. rufus would vary from 58 in Trinidad and Venezuela (Warner et al. 1974) to 64 in Pernambuco, with 62 in Piauí. In this case, new karyotypic studies are necessary to confirm this variation and to determine whether the karyotype from Pernambuco is in fact from M. rufus or M. fluminensis, and thus to clarify the karyotypic variation of these two species.
Molossops temminckii. The karyotypic study carried out by Santos (2013), based on specimens collected in Itinga (MG), described three pairs of metacentric chromosomes. In contrast, the study by Morielle-Versute et al. (1996) for Minas Gerais found no metacentric chromosomes. This discrepancy resulted in differences in the number of submetacentric, subtelocentric, and acrocentric chromosomes between the karyotypes. No variations were observed in the description of the X chromosome. Since Santos (2013) only analyzed a single female, it was not possible to assess the Y chromosome.
Thus, the karyotypes of Molossops temminckii exhibit variations in the morphology of autosomal chromosomes within the state of Minas Gerais. Since the karyotype described by Morielle-Versute et al. (1996) lacks a precise description of the collection location, it is not possible to determine whether the variation observed relative to Santos (2013) is truly between the Cerrado and Atlantic Forest biomes, or merely within different areas of the Atlantic Forest in Minas Gerais.
Eumops perotis. Considering the studies by Corrêa (2016) and Morielle-Versute et al. (1996), Eumops perotis exhibited subtle karyotypic variation between the Amazon and Atlantic Forest regions, particularly in the position of the centromere in five pairs of autosomal chromosomes. Since Corrêa (2016) karyotyped only females, it was not possible to draw conclusions about the X chromosome. Considering the lack of records for Eumops perotis in much of central Brazil, the populations in the Amazon and Atlantic Forest biomes may be undergoing isolation. This potential isolation is supported by genetic studies on birds and bats across their distribution in Brazil, which have indicated similar patterns of differentiation and isolation (Martins et al. 2009; Pavan et al. 2011; Batalha-Filho et al. 2013).
Cynomops planirostris. We observed numerical and structural karyotypic variations, both in autosomal and sexual chromosomes, among three different populations of Cynomops planirostris occurring in the Amazon and the Atlantic Forest. The karyotypes for the northern Atlantic Forest (Leite-Silva et al. 2003) and the Amazon (Corrêa 2016) differed from the karyotype for the central Atlantic Forest (Santos 2013) in relation to FN. This variation is largely attributed to differences in the morphology of two pairs of autosomal chromosomes across the studies of these three populations.
The Y chromosome also showed a closer relationship between the populations of the Amazon and the northern Atlantic Forest, differing morphologically from the Y chromosome described for the central region of the Atlantic Forest by Santos (2013). Notably, the Y chromosome described by Corrêa (2016) for the Amazon has a completely heterochromatic short arm, whereas the Y chromosome described by Leite-Silva et al. (2003) for the northern Atlantic Forest lacks constitutive heterochromatin. This variation may indicate a degree of isolation between these populations.
The chromosomal variations observed among the karyotypic descriptions of molossids collected in Brazil may result from several chromosomal rearrangement processes, such as insertions/deletions or pericentric inversions. For instance, in Eumops perotis, the main differences between studies were related to the position of the centromeres and/or the size of the chromosome arms. Additionally, these variations may be due to Robertsonian translocations, as suggested by the differences found in Molossops temminckii and Cynomops planirostris.
However, the processes and origins of the variations observed require further investigation. Improved banding techniques and the use of chromosomal probes are essential for understanding the evolution of the molossid karyotype throughout their distribution in Brazil. Sotero-Caio et al. (2017) and Leite-Silva et al. (2003) highlighted that in Molossidae, determining the fundamental number is challenging because the short arms of the chromosomes can be extremely small, complicating the distinction between subtelocentric and acrocentric forms. However, according to these authors, the detection of these differences is important, as they portray the mode of chromosomal evolution of Molossidae, which appears to be based mainly on pericentric inversions.
Leite-Silva et al. (2003) highlight that various studies suggest nucleolus organizing regions (NOR’s) are important markers for studies of chromosomal evolution in Chiroptera and emphasize their potential role in the chromosomal evolution of molossids. Analyzes carried out on Cynomops abrasus, Cynomops planirostris, and Molossops temminckii, revealed that none of these species share the same number of NOR-bearing chromosomes (Leite-Silva et al. 2003; Morielle-Versute et al. 1996).
Furthermore, Cynomops abrasus and C. planirostris exhibit variation in the number of chromosomes with constitutive heterochromatin. Cynomops planirostris shows constitutive heterochromatin on the short arm of the X chromosome and on five autosomal chromosomes, while C. abrasus and M. temminckii have constitutive heterochromatin on all autosomal chromosomes (Corrêa and Bonvicino 2016; Leite-Silva et al. 2003; Morielle-Versute et al. 1996). Previously, these three species were classified under the genus Molossops, given that the hypothesis of intrageneric variation considered by Leite-Silva et al. (2003) and Morielle-Versute et al. (1996) currently also extends to an intergeneric variation.
It is important to highlight that none of the karyotypes found in the literature refer to a type specimen or a specimen collected at the type locality of these species. This highlights the need for investment in karyotypic studies to establish a “type karyotype” for each species and to subsequently describe the diversity and karyotypic evolution among species.
Influences of geoclimatic changes on the karyotypic variation of Brazilian molossid bats. Different studies indicate that Brazilian biomes experienced a highly dynamic history during the Tertiary and Quaternary periods, shaped by various climatic events that led to complex processes of retraction and expansion over time. These events influenced Brazilian biomes and were crucial in shaping the current floristic and faunal composition of Brazil (Machado et al. 2018; Silveira et al. 2019; Werneck et al. 2012).
Phylogeographic studies based on molecular data with certain groups of rodents and marsupials, as well as suboscine birds occurring in the Amazon and the Atlantic Forest, have identified similar connection routes between these biomes over time. The oldest connections are dated to the middle to late Miocene through central Brazil and Chaco region. Most recent connections likely occurred through the Cerrado and Caatinga in the northeastern Brazil from the Pliocene to the Pleistocene, driven by Quaternary climate changes that facilitated the expansion of gallery forests through these areas (Batalha-Filho et al. 2013; Costa 2003).
According to Werneck et al. (2012), during the Last Interglacial, around 120,000 years ago, the climate was hotter and drier, likely promoting an expansion of the Cerrado into areas including the northern Amazon and the eastern coast of South American. Between the Last Interglacial and the Last Glacial Maximum, the Cerrado underwent a process of retraction, reaching its smallest extent during this period. Following this, the Cerrado began to expand again until the Middle Holocene, approximately 6,000 years ago, when it started to stabilize into its current form.
According to a study using molecular data from the vampire bat Desmodus rotundus, which is widely distributed across all Brazilian biomes, genetically structured populations were found for the southern Atlantic Forest, northern Atlantic Forest, Amazon, Cerrado, Pantanal, and Central America. This study indicates that Atlantic Forest populations separated from those in the Amazon and Cerrado during the Pleistocene, which is consistent with the emergence of a dry strip separating the Atlantic Forest from the Amazon during that period (Martins et al. 2009). The authors considered that there are no identifiable physical barriers to dispersal and gene flow within this bat distribution range, suggesting that the population structuring may be influenced by ecological separation barriers.
The same type of study conducted by Pavan et al., (2011) suggested that Carollia perspicillata and C. brevicauda, from the Phyllostomidae, may have appeared in the Amazon region during the Pleistocene approximately 700,000 years ago. It was also observed that C. perspicillata has two main evolutionary lineages that may have diverged during the Pleistocene: one lineage extends from Bahia to Paraná in the southern Brazilian Atlantic Forest, while the other, more geographically widespread, is distributed across the northern part of the Atlantic Forest, Cerrado, and Amazon, as well as in other biomes of South and Central America (Pavan et al. 2011).
In a comparative phylogeographic analysis of island and continental bat species of the genus Molossus, Loureiro et al. (2020a) demonstrated the influence of the Amazon River as a dispersal barrier, as well as the impact of ecological factors and vegetation formations on the genetic structuring of these species in South America.
Thus, recent studies indicate that, although bats are capable of true flight, they are still affected by geographic distance and ecological variants, such as the distinction of vegetation between biomes (Pavan et al. 2011; Morales et al. 2018; Loureiro et al.2020a).
The results of this study reveal that, despite the limited number of karyotypic studies on molossids, it was possible to observe variations in the species between karyotypes from different biomes, and even within the same biome. The Molossidae family, which dates back 50 to 31 million years, began its diversification into Neotropical clades approximately 20 million years (Amador et al. 2016). This study underscores the critical need for increased investment in karyotypic research on molossid bats to better understand interspecific and intraspecific variations and the evolutionary trajectory of their karyotypes. Above all, this investment is necessary due to the presence of population variations and cryptic species, as seen with Molossus fluminensis and M. rufus, so that conservation and management plans appropriate to the group can be carried out.
Acknowledgments
We would like to thank the support and development institutions Universidade Federal do Rio de Janeiro, PIBIC/UFRJ for providing scientific initiation and that financed the production of this work. LMP is partially supported by FAPERJ- E-26/210.312/2021, COLBIO by the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro. Financial support was also provided to NSVL by the PDR - E39/2021; Grant E- 6/200.957/2023) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Brazilian Government). We are very grateful to Dr. R. Leonan and R. Gregorin for help in the cited literature.
Literature cited
Amador, L. I., et al. 2016. Bat systematics in the light of unconstrained analysis of a comprehensive molecular supermatrix. Journal of Mammalian Evolution 25:37-70.
Ao, L., et al. 2006. Karyotype relationships of six bat species (Chiroptera, Vespertilionidae) from China revealed by chromosome painting and G-banding comparison. Cytogenetic and Genome Research 115:145-153.
Baker, R. J. 1970. Karyotypic trends in bats. Pp 65-96, in Biology of bats. Vol I (Wimsatt, W. A. ed.). Academic Press Inc., New York, U.S.A.
Barros, M. A. S. 2014. First record of Molossus molossus (Pallas, 1766) (Mammalia: Chiroptera) in the state of Rio Grande do Norte, northeastern Brazil. CheckList 10:1520-1524.
Batalha-Filho, H., J. Fjeldså, P. H. Fabre, and C. Y. Miyaki. 2013. Connections between the Atlantic and the Amazonian Forest avifauna represent distinct tropical events. Journal of Ornithology 154:41-50.
Beven, S., E. F. Connor, and K. Beven. 1984. Avian biogeography in the Amazon basin and the biological model of diversification. Journal of Biogeography 11:383-399.
Bonvicino, C. R., And M. Weksler. 1998. A new species of Oligoryzomys (Rodentia, Sigmodontinae) from northeastern and central Brazil. Zeitschrift fur Saugetierkunde 63: 90-103.
Brandão, V. C. 2015. Estudocitogenético em Molossus molossus (Chiroptera, Molossidae) do bioma Amazônia. Monografia. Instituto Federal de Educação, Ciência e Tecnologia do Pará – IFPA.
Corrêa, M. M. O. 2016. Citotaxonomia de quirópteros na Amazônia brasileira e filogeografia de espéciespotenciaishospedeiras de zoonoses. Tese de Doutorado. Instituto Oswaldo Cruz.
Corrêa, M. M. de O., and C. R. Bonvicino. 2016. C-banding variation in some Brazilian Amazon bats (Chiroptera). Boletim da Sociedade Brasisleira de Mastozoologia 77:105-116.
Costa, L. P. 2003. The historical bridge between the Amazon and the Atlantic Forest of Brazil: a study of molecular phylogeography with small mammals. Journal of biogeography 30:71-86.
Christoff, A. U., et al. 2000. Description of a new species of Akodon (Rodentia: Sigmodontinae) from southern Brazil. Journal of Mammalogy 81:838-851.
de Lemos Pinto, M. M. P., et al. 2012. Cytotaxonomy of the subgenus Artibeus (Phyllostomidae, Chiroptera) by characterization of species-specific markers. Comparative Cytogenetics 6:17.
Eger, J. L. 1977. Systematics of the genus Eumops (Chiroptera: Molossidae). Life Sciences Contributions Royal Ontario Museum 110:1-69.
Eger, J. L. 2008. Family Molossidae P. Gervais, 1856. Pp 399-440, in Mammals of South America, Volume 1: Marsupials, Xenarthrans, Shrews and Bats (A. L. Gardner, ed.). University of Chicago Press, Chicago.
Eick, G. N., D. S. Jacobs, F. Yang, and M. Volleth. 2007. Karyotypic differences in two sibling species of Scotophilus from South Africa (Vespertilionidae, Chiroptera, Mammalia). Cytogenetic and Genome Research 118:72-77.
Faria, K. D. C. 2003. Análise citogenética comparative em espécies de morcegos dos gêneros Molossus (Molossidae), Artibeus, Platyrrhinus, Sturnira, Glossophaga, Phyllostomus e Carollia (Phyllostamide) Chiroptera (Mammalia). Dissertação de Mestrado. Universidade Estadual Paulista, Instituto de Biociências, Letras e Ciências Exatas. http://hdl.handle.net/11449/92536.
Faria, K. C., and E. Morielle-Versute. 2006. Genetic relationships between Brazilian species of Molossidae and Phyllostomidae (Chiroptera, Mammalia). Genetica 126:215-225.
Garbino, G. S. T., et al. 2022. Updated checklist of Brazilian bats: versão 2022. Comitê da Lista de Morcegos do Brasil - CLMB. Sociedade brasileira para o Estudo de Quirópteros (Sbeq). https://www.sbeq.net/lista-de-especies. Accessed 03 March 2023.
Granjon, L., and G. Dobigny. 2003. The importance of cytotaxonomy in understanding the biogeography of African rodents: Lake Chad murids as an example. Mammal Review 33:77-91.
Gomes, A., et al. 2010. Biogeographical karyotypic variation of Rhinophylla fischerae (Chiroptera: Phyllostomidae) suggests the occurrence of cryptic species. Comparative Cytogenetics 4:79-85.
Gregorin, R., and A. Cirranello. 2016. Phylogeny of Molossidae Gervais (Mammalia: Chiroptera) inferred by morphological data. Cladistics 32:2-35.
Leal, A. O. S. 2012. Identificação taxonômica e caracterização citogenética das espécies de morcegos coletadas no município de Picos-Piauí. Monografia. Universidade Federal do Piauí.
Leite‐Silva, C., et al. 2003. Karyotypic characterization of the bat species Molossus ater, M. molossus and Molossops planirostris (Chiroptera, Molossidae) using FISH and banding techniques. Hereditas 138:94-100.
Levan, A., K. Fredga, and A. A. Sandberg. 1964. Nomenclature for centromeric position on chromosomes. Hereditas 52:201-220.
Loureiro, L. O., R. Gregorin, and F. A. Perini. 2018. Diversity, morphological phylogeny, and distribution of bats of the genus Molossus E. Geoffroy, 1805 (Chiroptera, Molossidae) in Brazil. Zoosystema 40:425-452.
Loureiro, L. O., M. D. Engstrom, and B. K. Lim. 2020a. Comparative phylogeography of mainland and insular species of Neotropical molossid bats (Molossus). Ecology and evolution 10:389-409.
Loureiro, L. O., M. D. Engstrom, and B. K. Lim. 2020b. Single nucleotide polymorphisms (SNPs) provide unprecedented resolution of species boundaries, phylogenetic relationships, and genetic diversity in the mastiff bats (Molossus). Molecular Phylogenetics and Evolution 143:106-690.
Machado, A. F. P., et al. 2018. Atlantic forests to the all-Americas: Biogeographical history and divergence times of Neotropical Ficus (Moraceae). Molecular Phylogenetics and Evolution 122:46-58.
Martins, F. M. 2011. Historical biogeography of the Brazilian Atlantic Forest and the Carnaval–Moritz model of Pleistocene refugia: what do phylogeographical studies tell us? Biological Journal of the Linnean Society 104:499-509.
Martins, F. M., et al. 2009. Phylogeography of the common vampire bat (Desmodus rotundus): marked population structure, Neotropical Pleistocene vicariance and incongruence between nuclear and mtDNA markers. BMC Evolutionary Biology 9:1-13.
Mendes, S. B., et al. 2020. First record of Cynomops planirostris (Peters, 1865) (Chiroptera, Molossidae) from Maranhão state, Brazil, based on morphological and molecular data. Brazilian Journal of Biology 80:405-409.
Morales, A. E., M. De‐la‐Mora, and D. Piñero. 2018. Spatial and environmental factors predict skull variation and genetic structure in the cosmopolitan bat Tadarida brasiliensis. Journal of Biogeography 45:1529-1540.
Morielle-Versute, E., M. Varella-Garcia, and V. A. Taddei. 1996. Karyotypic patterns of seven species of molossid bats (Molossidae, Chiroptera). Cytogenetic and Genome Research 72:26-33.
Moratelli, R., andE. Morielle-Versute. 2007. Métodos e aplicações da citogenética na taxonomia de morcegos brasileiros. Pp 197-218, in Morcegos do Brasil (Reis, N. R., A. L. Peracchi, W. A. Pedro, and I. P. Lima, eds.). Editora UEL, Londrina, Brazil.
Nunes, H., et al. 2013. First and easternmost record of Molossops temminckii (Burmeister, 1854) (Chiroptera: Molossidae) for the state of Paraíba, northeastern Brazil. CheckList 9:436-439.
Okumura Finato, A., et al. 2000. Intrachromosomal distribution of telomeric repeats in Eumops glaucinus and Eumops perotis (Molossidae, Chiroptera). Chromosome Research 8:563-569.
Palacios-Mosquera, L., et al. 2020. Systematics and taxonomy of Platyrrhinus chocoensis (Chiroptera: Phyllostomidae) based on morphometric and genetic analyses: implications for biogeography and conservation. Mammalian Biology 100:113-124.
Paglia, A. P., et al. 2012. Lista Anotada dos Mamíferos do Brasil 2ª Edição/Annotated Checklist of Brazilian Mammals. Occasional Papers in Conservation Biology 6:1-76.
Pavan, A. C., et al. 2011. Patterns of diversification in two species of short-tailed bats (Carollia Gray, 1838): the effects of historical fragmentation of Brazilian rain forests. Biological Journal of the Linnean Society 102:527-539.
Peracchi, A. L., M. R. Nogueira, I. P. Lima. 2011. Novos achegos à lista dos quirópteros do município de Linhares, estado do Espírito Santo, sudeste do Brasil (Mammalia, Chiroptera). Chiroptera Neotropical 17:842-852.
Rocha, P. A., et al. 2010. Morcegos (Mammalia, Chiroptera) capturados no Campus da Universidade Federal de Sergipe, com oito novos registros para o estado. Biota Neotropica 10:183-188.
Santos, B. A. 2013. Diversidade cariotípica de morcegos (Mammalia: Chiroptera) do Cerrado, Mata Atlântica e áreas de transição do Vale do Rio Jequitinhonha, Minas Gerais, Brasil. Monograph for the bachelor's degree in Ecology. Veiga de Almeida University.
Santos, T. C. M., et al. 2015. New records of Cynomops planirostris (Peters, 1865) (Chiroptera, Molossidae) for the state of Amazonas and its updated distribution in Brazil. CheckList 11:1787-1787.
Santos, T., G. P. Lopes, R. M. Rabelo, and T. C. Giannini. 2019. Bats in three protected areas of the Central Amazon Ecological Corridor in Brazil. Acta Chiropterologica 21:425-442.
Silva, F. P., et al. 2023. Pleistocene distribution of MacConnell’s Bat (Phyllostomidae) suggests intermittent connections between Amazonia and Atlantic Forest. Therya 14:55.
Silveira, M. H. B., R. Mascarenhas, D. Cardoso, and H. Batalha-Filho. 2019. Pleistocene climatic instability drove the historical distribution of forest islands in the northeastern Brazilian Atlantic Forest. Palaeogeography, Palaeoclimatology, Palaeoecology 527: 67-76.
Simmons, N. B., and A. L. Cirranello. 2023. Bat Species of the World: A taxonomic and geographic database. www.batnames.org. Accessed 03 June 2023.
Souza, J. C., et al. 2016. Molossus rufus (E. Geoffroy, 1805) (Mammalia, Chiroptera): Geographic distribution and first record for the state of Sergipe, northeastern Brazil. Neotropical Biology and Conservation 11:184.
Sotero-Caio, C. G., R. J. Baker, and M. Volleth. 2017. Chromosomal evolution in Chiroptera. Genes 8:272. https://doi.org/10.3390/genes8100272.
Taylor PJ, Lim BK, Pennay M, Soisook P, Loureiro LO, Moras LM, Kingston T. 2019. Family Molossidae (Free-tailed bats). Pp. 598-673, in Handbook of the Mammals of the World, vol. 9 - Bats (Wilson, D. E., and R. A. Mittermeier, eds.). Barcelona, Lynx Editions.
Thompson, K. W. 1979. Cytotaxonomy of 41 species of Neotropical Cichlidae. Copeia 1979:679-691.
Torres, J. M., et al. 2020. First record of Eumops perotis (Schinz, 1821) (Chiroptera, Molossidae) from the Cerrado of Mato Grosso do Sul, Central-West Brazil. CheckList 16:759-764.
Varella-Garcia, M., E. Morielle-Versute, and V. A. Taddei. 1989. A survey of cytogenetic data on Brazilian bats. Revista Brasileira de Genética 12:761-793.
Varella-Garcia, M., and V. A. Taddei. 1989. Citogenética de quirópteros: métodos e aplicações. Revista Brasileira de Zoologia 6:297-323.
Vaughan, T. A. 1966. Morphology and flight characteristics of molossid bats. Journal of Mammalogy 47:249-260.
Warner, J. W., et al. 1974. Karyotypic analyses of twenty-one species of molossid bats (Molossidae: Chiroptera). Canadian Journal of Genetics and Cytology 16:165-176.
Werneck, F. P., et al. 2012. Climatic stability in the Brazilian Cerrado: implications for biogeographical connections of South American savannas, species richness and conservation in a biodiversity hotspot. Journal of Biogeography 39:1695-1706.
Wilson, D. E. and R. A. Mittermeier (eds.) 2019. Handbook of the Mammals of the World. Vol. 9. Bats. Lynx Edicions. Barcelona, Spain.
Associated editor: Sergio Solari
Submitted: June 4, 2024; Reviewed: June 24, 2024
Accepted: August 18, 2024; Published on line: September 3, 2024
Table 1. Species of Molossidae recorded in Brazil and the respective biomes in which they occur. Amazon (Am); Atlantic Forest (AF); Cerrado (Ce); Caatinga (Ca); Pantanal (Pt); Pampa (Pp).
|
Species |
Biomes |
References |
|
Cynomops abrasus |
Am, AF, Ce, Ca, Pt |
Paglia et al. 2012 |
|
Cynomops greenhalli |
Am, Ca |
Paglia et al. 2012 |
|
Cynomops milleri |
Am |
Moras et al. 2018 |
|
Cynomops mastivus |
Am |
Moras et al. 2016 |
|
Cynomops planirostris |
Am, AF, Ce, Ca, Pt |
Santos et al. 2015; Mendes et al. 2020 |
|
Eumops auripendulus |
Am, AF, Ce, Ca, Pt |
Eger 1977, 2008 |
|
Eumops bonariensis |
AF, Ce, Pp |
Eger 2008; Bordignon, 2006; Bernardi et al. 2009 |
|
Eumops chimaera |
AF |
Gregorin et al. 2016 |
|
Eumops dabbenei |
Pt |
Fischer et al. 2015 |
|
Eumops delticus |
Am, AF, Ce |
Eger 2008; Silva et al. 2013 |
|
Eumops glaucinus |
Am, AF, Ce, Ca, Pt |
Paglia et al. 2012 |
|
Eumops hansae |
Am, AF, Ce |
Paglia et al. 2012 |
|
Eumops maurus |
Am, AF, Ce |
Eger 2008; Sodré et al. 2008; Díaz, 2011 |
|
Eumops patagonicus |
AF, Pp, Pt |
Bernardi et al. 2009; Bordignon et al. 2011; Carvalho et al. 2017 |
|
Eumops perotis |
Am, AF, Ce, Ca, Pt |
Torres et al. 2020 |
|
Eumops trumbulli |
Am |
Paglia et al. 2012 |
|
Molossops neglectus |
Am, AF |
Althoff et al. 2018 |
|
Molossops temminckii |
Am, AF, Ce, Ca, Pt |
Nunes et al. 2013 |
|
Molossus aztecus |
Am, AF, Ce, Ca, Pt |
Loureiro et al. 2018 |
|
Molossus coibensis |
Am, AF, Ce, Ca |
Loureiro et al. 2018 |
|
Molossus currentium |
Am, Pt |
Paglia et al. 2012; Loureiro et al. 2018 |
|
Molossus fluminensis |
AF, Ce, Pt |
Loureiro et al. 2020a |
|
Molossus molossus |
Am, AF, Ce, Ca, Pt, Pp |
Rocha et al. 2010; Barros et al. 2014; Loureiro et al. 2018 |
|
Molossus pretiosus |
Ce, Pt |
Loureiro et al. 2018 |
|
Molossus rufus |
Am, AF, Ce, Ca, Pt |
Souza et al. 2016; Loureiro et al. 2018 |
|
Neoplatymops mattogrossensis |
Am, AF, Ce, Ca |
Novaes et al. 2013 |
|
Nyctinomops aurispinosus |
AF, Ce, Ca |
Oliveria et al. 2019 |
|
Nyctinomops laticaudatus |
Am, AF, Ce, Ca, Pt, Pp |
Paglia et al. 2012 |
|
Nyctinomops macrotis |
Am, AF, Ce, Ca, Pt |
Rocha et al. 2015 |
|
Promops centralis |
Am, AF, Ce, Ca, Pt |
Hintze, et al. 2020 |
|
Promops nasutus |
Am, AF, Ce, Ca, Pt, Pp |
Paglia et al. 2012 |
|
Tadarida brasiliensis |
Am, AF, Ce, Ca, Pt, Pp |
Tavares et al. 2008 |
Figure 1. Map of the distribution and location of karyotypic studies of Molossus molossus. Black dots: occurrence records. Blue Crosses: records that have karyotypic data described.

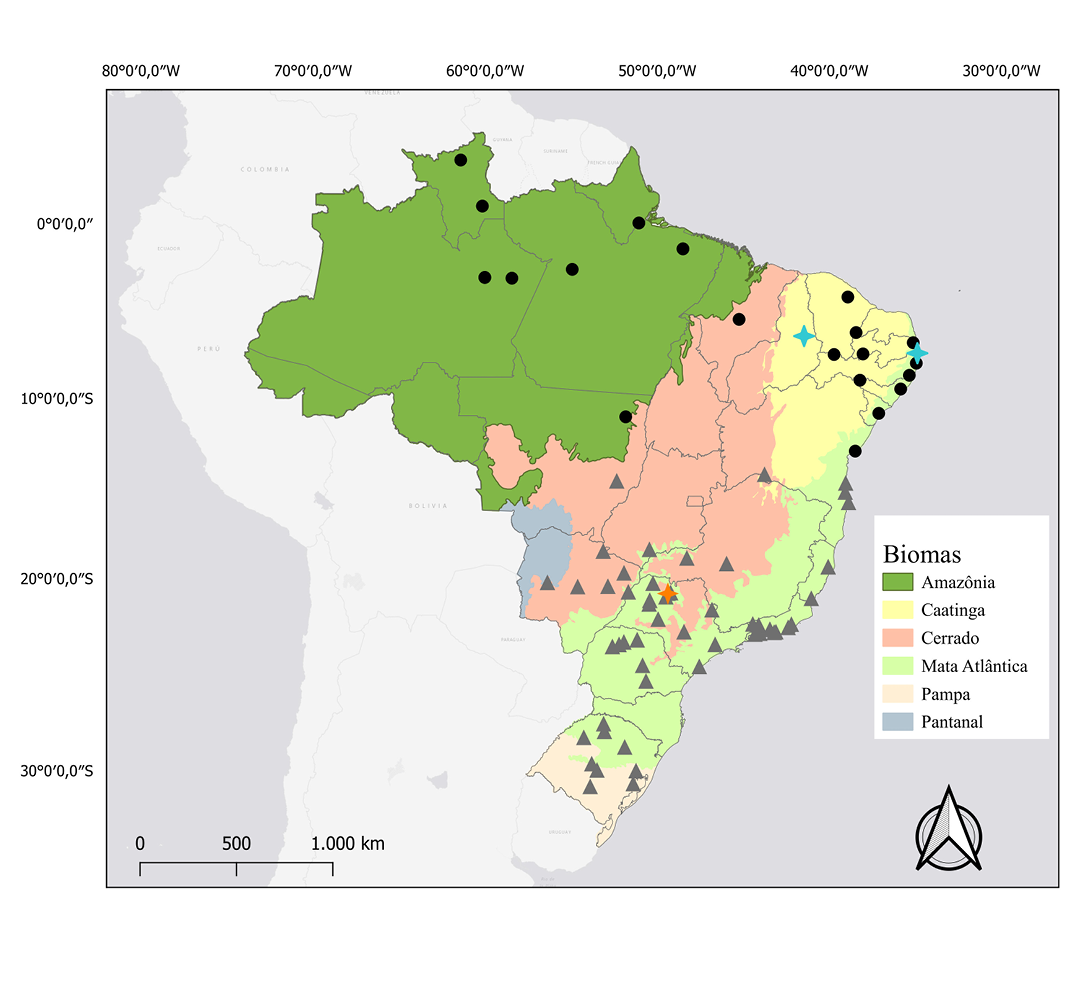
Figure 2. Map of the distribution and location of karyotypic studies of Molossus rufus and M. fluminensis. Black dots: occurrence records of M. rufus. Blue Crosses: records that have karyotypic data described for M. rufus. Gray triangle: potential occurrence records of M. fluminensis. Orange Crosses: records that have karyotypic data described, potentially, for M. fluminensis.
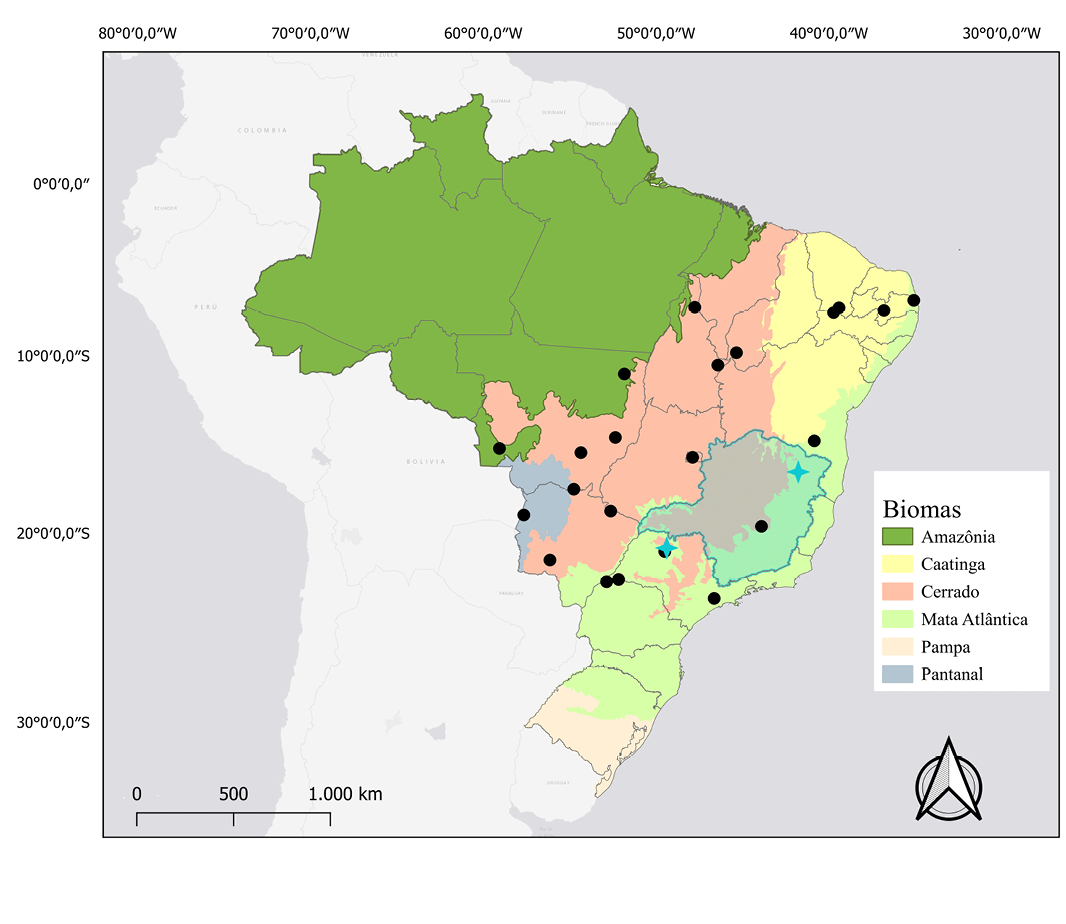
Figure 3. Map of the distribution and location of karyotypic studies of Molossops temminckii. Black dots: occurrence records. Blue Crosses: records that have karyotypic data described.
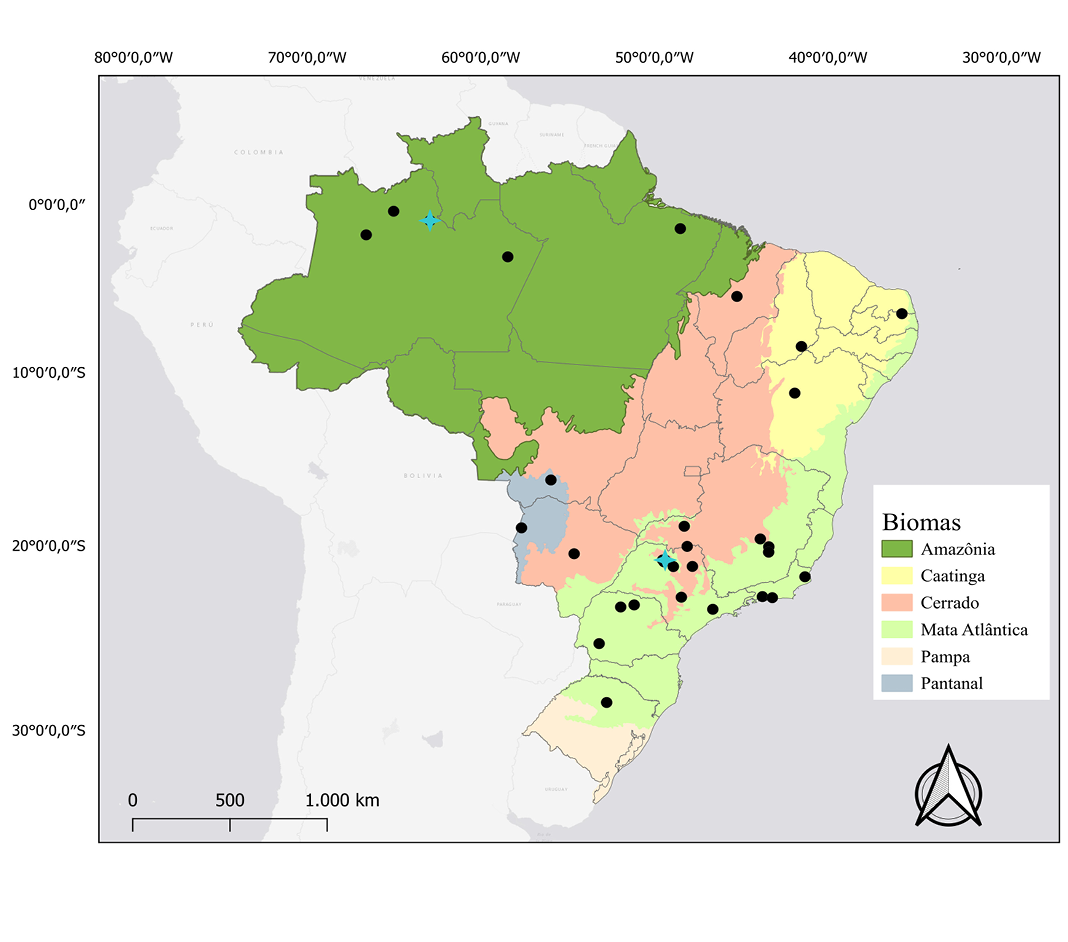
Figure 4. Map of the distribution and location of karyotypic studies of Eumops perotis. Black dots: occurrence records. Blue Crosses: records that have karyotypic data described.
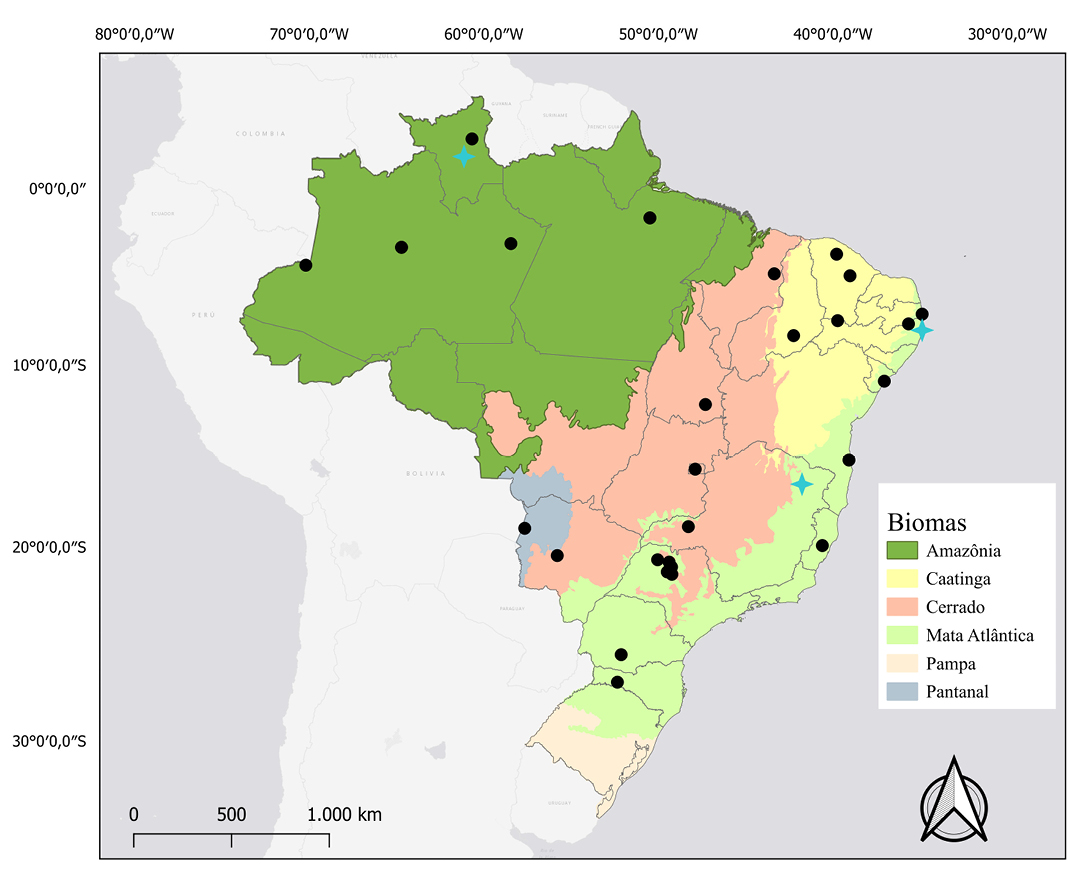
Figure 5. Map of the distribution and location of karyotypic studies of Cynomops planirostris. Black dots: occurrence records. Blue Crosses: records that have karyotypic data described.
Table 2. Karyotypic data of fourteen species of molossids that occur in Brazil. Diploid number (2n); Fundamental Number (FN); synthetic nucleotide 5-bromo-2-deoxyuridine (BrdU); Band C (C); G Band (G); conventional staining (Giemsa-); Nucleolus organizing regions (NOR); Fluorescence in situ hybridization (FISH). Biomes: Amazon (Am); Atlantic Forest (AF); Cerrado (Ce); Caatinga (Ca); Pantanal (Pt); Pampa (Pp).
|
Species |
2n |
Fn |
Method |
Biome |
References |
|
Cynomops abrasus |
34 |
60 |
Giemsa |
- |
27 |
|
34 |
64 |
C and G |
FA |
20 |
|
|
Cynomops greenhalli |
34 |
60 |
Giemsa |
- |
2, 9, 16, 27 |
|
Cynomops planirostris |
34 |
64 |
Giemsa |
FA |
22 |
|
34 |
60 |
G, C, NOR and FISH |
FA |
14,15 |
|
|
34 |
60 |
C |
Am |
5 |
|
|
Eumops auripendulus |
42 |
60 |
Giemsa |
- |
27 |
|
42 |
62 |
Giemsa |
- |
25 |
|
|
Eumops glaucinus |
40 |
64 |
G, C and NOR |
FA |
12, 18, 20, |
|
40 |
64 |
Giemsa |
- |
27 |
|
|
38 |
64 |
Giemsa |
- |
27 |
|
|
Eumops hansae |
48 |
58 |
Giemsa and FISH |
Am |
5 |
|
Eumops perotis |
48 |
54 |
Giemsa |
- |
24 |
|
48 |
56 |
Giemsa |
- |
1, 26 |
|
|
48 |
58 |
C and G |
FA |
12, 20 |
|
|
48 |
58 |
C |
Am |
5 |
|
|
Molossops temminckii |
42 |
56 |
Giemsa |
- |
9 |
|
42 |
56 |
Giemsa |
FA |
22 |
|
|
48 |
68 |
C and G |
FA |
10 |
|
|
Molossus molossus |
48 |
56 |
C, G and NOR |
Pa, FA |
3, 7, 17 |
|
48 |
58 |
Giemsa |
- |
27 |
|
|
48 |
64 |
G, C, RON, BrdU and FISH |
FA |
10, 15, 20, 21, 18 |
|
|
48 |
64 |
C, G and RON |
Am |
4, 5, 6 |
|
|
Molossus rufus |
48 |
58 |
Giemsa |
- |
27 |
|
48 |
60 |
Giemsa |
FA |
17, 25 |
|
|
48 |
62 |
Giemsa |
Ce, Ca |
8, 13, 23 |
|
|
48 |
64 |
G, C, NOR, BrdU and FISH |
FA |
10, 11, 15, 20, 21 |
|
|
Nyctinomops laticaudatus |
48 |
64 |
C and G |
FA |
20 |
|
Promops centralis |
48 |
58 |
Giemsa |
- |
27 |
|
Promops nasutus |
40 |
54 |
Giemsa |
- |
26 |
|
Tadarida brasiliensis |
48 |
56 |
G |
- |
2, 27 |
|
|
48 |
58 |
Giemsa |
- |
27 |
References: 1. Baker (1970); 2. Baker et al. (1982)a; 3. Baker and Lopez (1970); 4. Brandão (2015); 5. Corrêa (2016); 6. Corrêa and Bovicino (2016); 7. Cristoff and Freitas (1987)a; 8. Dantas (2004)c; 9. Gardner (1977)b; 10. Faria (2003); 11. Faria and Morielle-Versute (2006); 12. Finato (2000); 13. Leal (2012); 14. Leite-Silva et al. (2000) b; 15. Leite-Silva et al.(2003); 16. Linares and Kiblisky (1969)b; 17. Lopes (1978)a; 18. Moratelli et al. (2000)b; 19. Morielle et al.(1988)a; 20. Morielle-Versute et al. (1996); 21. Morielle-Versute and Varella-Garcia (1994); 22. Santos (2013); 23. Sousa (2007)c; 24. Painter (1925)b; 25. Toledo (1973)a; 26. Wainberg (1966)b; 27. Warner et al. (1974). The citation's final letters indicate the cited author and data source: a - Varella-Garcia and Taddei (1989); b - Moratelli et al. (2007)); c - Leal (2012).