THERYA, 2024, Vol. 15(3):259-267 DOI:10.12933/therya-24-6135 ISSN 2007-3364
Baird’s Tapir (Tapirus bairdii) avoid human settlements and roads while searching for water in community-owned forests from the Calakmul region
Jonathan O. Huerta-Rodríguez1, Itzel Poot-Sarmiento2, Alan Duarte-Morales3, Lizzi V. Martínez-Martínez4, and Rafael Reyna-Hurtado1*
1 El Colegio de la Frontera Sur Unidad Campeche. Avenida Rancho s/n, Polígono 2, CP. 24500, Lerma. Campeche, México. Email: jonathan.huerta@posgrado.ecosur.mx (JOH-R); rreyna@ecosur.mx (RR-H).
2 Zion Consultores, Andador Becan, Plan Chac, CP. 24000, Campeche. Campeche, México.. Email: al051903@uacam.mx (IP-S).
3 Centro de Desarrollo Sustentable, Universidad Autonoma de Campeche, CP. 24000, Campeche. Campeche, México. Email: al059027@uacam.mx (AD-M).
4 Reserva de la Biosfera de Calakmul, Comisión Nacional de Áreas Naturales Protegidas, CalleViveros s/n, Zoh Laguna, CP. 24644, Calakmul. Campeche, México. Email: lizzi.martinez@posgrado.ecosur.mx (LVM-M).
*Corresponding author: https://orcid.org/0000-0003-4382-642x.
Baird’s tapir (Tapirus bairdii) populations are declining because of deforestation, fragmentation, poaching, and vehicle collisions. Tapirs play an ecological role as seed dispersers and seed predators; therefore, their loss will impact plant communities. This species prefers large areas with continuous native forest where human pressure is low and with permanent water bodies. In the Calakmul region of Southern Mexico, there are no permanent water bodies, only seasonal ponds called locally “aguadas”. These ponds have been crucial for tapir survival. Communal lands of the Calakmul region are important for tapirs, offering more surface water than the Calakmul Biosphere Reserve. Therefore, the objective of the present work was to determine the habitat features and anthropic factors influencing Baird’s tapir use of ponds on communal lands. Our hypothesis was that Baird's tapirs would use seasonal ponds that are farther from roads and human settlements with higher frequency than ponds near roads and human settlements. We set camera-trap stations for 50 days at 36 ponds on three communal lands from September to November 2022. We measured 3 habitat covariates: presence/absence of water, normalized differential vegetation index, and vegetation type. The disturbance covariates were Euclidean distance to roads and human settlements, and the sampling effort as the number of days stations were active. We evaluated the effect of these covariates in a single occupancy model, where we built detection histories in R v. 4. 2. 2 software. We obtained 60 independent records of Baird’s tapir at 27 out of the 36 ponds with a sampling effort of 1,599 camera trap nights. For tapir detection, the effort had a positive effect (β = 0.34 + 0.19, Wi = 0.66, p = 0.08). Distance to roads had the strongest effect (positive) on Baird’s tapir occupancy (β = 1.2 + 1.27, Wi = 0.39, p = 0.34), while Distance to human settlements also had a positive effect (β = 0.49 + 0.65, Wi = 0.17, p = 0.45). On communal lands from the Calakmul region, tapirs are searching for water sources on ponds far from roads and settlements to avoid potential conflicts with humans. Although tapirs are not hunted, other factors associated with villages and roads might encourage tapirs to avoid these areas. Communal lands have greater water availability in ponds than in the protected area, so therefore we recommend the creation of communal protected areas to preserve the remaining forests outside the reserve. With the arrival of mega-projects like the Maya train, the economic development of the study region will likely increase. For this reason, it’s of great importance that wildlife passages remain available and that mega-projects assure the free movement of tapirs to ponds in the Calakmul region.
Las poblaciones de tapir centroamericano (Tapirus bairdii) están disminuyendo globalmente por la deforestación, fragmentación, cacería y atropellamientos. Los tapires son dispersores y depredadores de varias semillas; por lo tanto, su extinción afectará a las comunidades vegetales. Esta especie prefiere extensiones grandes de bosque nativo, con presión humana baja y cuerpos de agua perennes. En la región de Calakmul en el sur de México no existen cuerpos de agua perennes, y el agua se almacena en depositos temporales llamados localmente aguadas, estas aguadas son vitales para la supervivencia del tapir. En la región de Calakmul, los ejidos son importantes para los tapires, pues hay mayor disponibilidad de agua en comparación con la Reserva de la Biósfera de Calakmul. Por lo tanto, el objetivo de este trabajo fue determinar las características del hábitat y los factores antrópicos que influyen en la ocupación del tapir centroamericano en aguadas de ejidos. La hipótesis fue que el tapir centroamericano utiliza aguadas alejadas de caminos y poblados con mayor frecuencia que las aguadas cercanas a caminos y poblados. Colocamos 36 estaciones de fototrampeo por 50 días, una por aguada en tres ejidos en el periodo de septiembre-noviembre de 2022. Medimos 3 covariables del hábitat: presencia/ausencia de agua, índice de vegetación diferencial normalizado y tipo de vegetación y 2 covariables de perturbación: la distancia Euclidiana a carreteras y poblados, y el esfuerzo de muestreo como número de días activos de las estaciones. Evaluamos el efecto de estas covariables con modelos de ocupación simples, donde construimos historias de detección en R v. 4. 2. 2. Registramos 60 eventos independientes del tapir en 27 de las 36 aguadas con un esfuerzo de muestreo de 1,599 noches-trampa. El esfuerzo de muestreo tuvo un efecto positivo (β = 0.34 + 0.19, Wi = 0.66, p = 0.08) en la detección del tapir. La distancia a caminos fue la covariable con mejor ajuste, con un efecto positivo en la ocupación del tapir (β = 1.2 + 1.27, Wi = 0.39, p = 0.34), seguida por la distancia a los poblados (β = 0.49 + 0.65, Wi = 0.17, p = 0.45). En los ejidos de la región de Calakmul los tapires van a aguadas lejos de caminos y poblados con mayor frecuencia que a aguadas cerca de caminos y poblados, sugiriendo que los tapires pueden estar evitando conflictos potenciales con los humanos. Aunque no son cazados frecuentemente, puede haber otros factores asociados a los pueblos y caminos que hacen que los tapires eviten ciertas aguadas. Como los ejidos tienen mayor disponibilidad de agua es necesario estimular la creación de reservas comunitarias, para conservar los bosques remanentes. Con los megaproyectos como el tren Maya, el desarrollo económico de la región de Calakmul aumentará. Por ello, es esencial que la fauna silvestre tenga acceso a las aguadas y que no se interrumpa su movimiento en la región de Calakmul.
Keywords: Communal lands; detection; occupancy; ponds; protected areas.
© 2024 Asociación Mexicana de Mastozoología, www.mastozoologiamexicana.org
Introduction
In México, tropical rainforests are among the major reservoirs of biodiversity. They once covered 9 % of the country, but over two-thirds have been cleared (Calderón-Aguilera et al. 2012). In addition, the loss of wildlife species that have an impact on the structure and composition of vegetation is affecting negatively the dynamics of the remaining ecosystem (Calderón-Aguilera et al. 2012; Trepel et al. 2024).
One of the species whose populations are declining in the tropics (García et al. 2016) is Baird’s tapir (Tapirus bairdii), a megaherbivore that feeds on shoots, leaves, and fruits from a wide variety of plants (Naranjo 2019). Tapirs play an ecological role as seed dispersers and seed predators of many plant species. They are capable of dispersing large seeds over long distances (O’Farrill et al. 2012; O’Farrill et al. 2013), contributing to the heterogeneity of the ecosystems (Trepel et al. 2024). Baird’s tapirs prefer areas with continuous native forest with different succession stages where the understory is dense and diverse, with low human pressure, and with permanent water bodies (Naranjo 2019; Schank et al. 2020; Falconi-Briones et al. 2022; Carrillo et al. 2015).
The Calakmul region, an area with significant amount of habitat for tapirs (Reyna-Hurtado and Tanner 2005), is part of the Maya Forest, the largest expanse of tropical forest in Mesoamerica, with nearly 30,000 km2 under protection in southeast México, northern Guatemala, and northern Belize (Reyna-Hurtado et al. 2022). The Calakmul region has most of its extension covered in karstic soil that absorbs the precipitation underground. The only water available on the surface are ponds called “aguadas”; these are used by many wildlife species, including tapirs and people from local communities, especially during the dry season (Reyna-Hurtado et al. 2022; Sandoval-Serés et al. 2016). These ponds have been crucial for the survival of Baird’s tapirs in this region where water is a limiting factor since they use water bodies to thermoregulate, flee from potential danger, relieve wounds (Naranjo 2019), and interact with their conspecifics (Reyna-Hurtado and Arias-Domínguez 2024).
For these reasons, the importance of ponds has been studied in this region, mainly in the Calakmul Biosphere Reserve (CBR), where several studies demonstrate that water availability in ponds influences the abundance and visitation of Baird’s tapir (Carrillo-Reyna et al. 2015; Reyna-Hurtado et al. 2019; Pérez-Cortez et al. 2012); and confirm tapir site fidelity to the ponds (Reyna-Hurtado and Arias-Domínguez 2024). Outside the CBR, only one study evaluated the importance of ponds for tapirs (Sandoval-Serés et al. 2016). Martinez et al. (2020) evaluated Baird’s tapir use of ponds in the Runaway Creek Nature Reserve in Belize, in response to human disturbance.
These studies highlight the importance that ponds have on Baird’s tapir population patterns and behavior. In this context, occupancy modelling is a valuable tool to assess species distribution while accounting for detection probabilities separately from occurrence probabilities, which can be modelled by covariates (Doser et al. 2022; MacKenzie et al. 2002).
These models have been used to evaluate the occurrence of Baird’s tapirs as a function of environmental and disturbance factors in several regions. Areas like the protected area of La Frailescana in Chiapas (de la Torre et al. 2018; Rivero et al. 2022), in communal lands from Los Chimalapas, Oaxaca (Pérez-Irineo and Santos-Moreno 2016), inin San Juan-La Selva Biological Corridor, Costa Rica (Cove et al. 2014) and in the Mesoamerican Biological Corridor in Panama (Meyer et al. 2020). Occupancy models have also been used to study habitat preferences in mountain tapir (Tapirus pinchaque) in the Tabaconas Namballe National Sanctuary in Perú (Mena et al. 2020), and in lowland tapirs (Tapirus terrestris) at Vale Natural Reserve, Brazil (Ferreguetti et al. 2017).
Despite the existing literature about the abundance and distribution of Baird’s tapir in different habitat types, little is known about the habitat features and anthropic factors that might be influencing the use of ponds outside protected areas. This is crucial for tapir conservation due to the habitat and water availability present in communal lands (Reyna-Hurtado et al. 2019; Carrillo et al. 2019; O’Farrill et al. 2014). Threats like vehicle collisions, retaliatory hunting, and deforestation (Serrano-Mac-Gregor et al. 2021; Naranjo 2018; Contreras-Moreno et al. 2013) for a specie with a low reproductive rate like Baird’s tapir (Pukazhenthi et al. 2013) are the reasons why its classified as endangered by the International Union for Conservation of Nature (IUCN; García et al. 2016; Schank et al. 2017; Reyna-Hurtado et al. 2019).
The objective of the present work was to determine the habitat features and anthropic factors influencing Baird’s tapir’s use of ponds on communal lands. Our hypothesis was that Baird's tapirs would use seasonal ponds that are farther from roads and human settlements with higher frequency than ponds near roads and human settlements.
Materials and methods
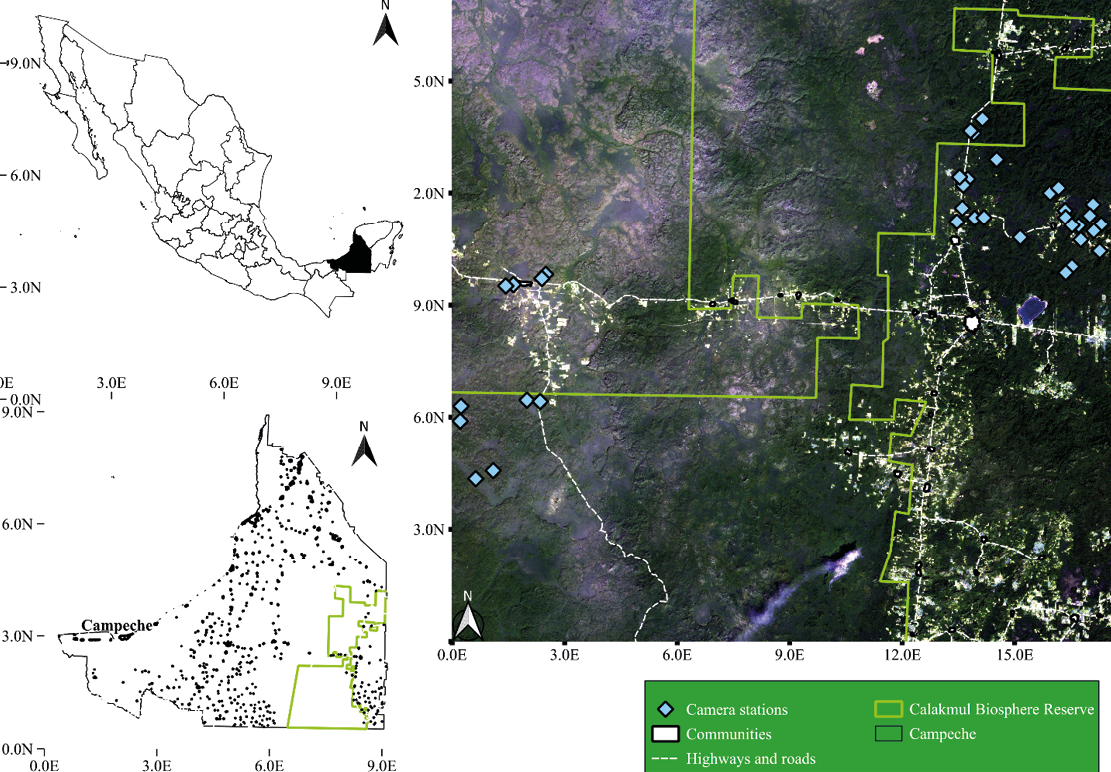
Study site. Our survey was carried out in the communal lands of Alvaro Obregon, Conhuas, and Nuevo Becal adjacent to the CBR, a protected area that preserves the largest tropical forest in México (Reyna-Hurtado and Tanner 2007). These communal lands were created in the 1970’s, when a colonization process encouraged by the government brought people from all over the country (Reyna-Hurtado and Tanner 2007). Today, 114 rural communities contain approximately 25,000 inhabitants (INE 2000) whose main activities are agriculture, livestock, logging, apiculture, sport hunting services and subsistence hunting (INE 2000; Reyna-Hurtado and Tanner 2007). The three community areas we studied are some of the largest communities of the region with 58,000 ha (Conhuas), 52,000 ha (Nuevo Becal) and 24,000 ha (Álvaro Obregón) respectively, of relatively well-conserved sub-perennial tropical forest (Pennington and Sarukhan 2005).
The climate is sub-humid tropical (Aw), mean annual precipitation is around 1100 mm, with 7 months of dry season, from November to May (Estrada-Medina et al. 2016; INE 2000). The main forest types are medium sub-perennial forest, low sub-perennial flooded forest, low semi-deciduous forest, and secondary forest (Reyna-Hurtado and Tanner 2005; Figure 1).
Camera trap survey. We deployed 36 stations with a single camera trap (Browning Strikeforce BTC-5HDX®) at 36 different ponds for 50 days from September to November 2022 after the main rainy season, when water is available in most of the ponds and other water bodies, so water is not a limiting factor. We placed 10 cameras in Conhuas, 12 in Zoh Laguna (Alvaro Obregon), and 14 in Nuevo Becal. To have spatial independence between records and considering the radius of the home range (4.1 km2) of a tapir from CBR (Reyna-Hurtado et al. 2016), camera stations were at least 1 km apart from each other. We set the cameras on trees at 40 or 50 cm above the ground facing north or south to avoid false triggers from sunlight. We programmed the cameras to take 3 consecutive pictures with 1-second delay between shots and to be active 24 hours. At the end of the survey, all photos were collected, classified, and organized to create detection histories, where the independent records were the detections of a tapir in each camera station during the 10-day interval of each occasion. These detections are represented with 1 and the non-detections with 0 for the creation of detection histories, using the package CamtrapR in R v. 4. 2. 2 software (Reyna-Hurtado et al. 2019).
Habitat and disturbance covariates. We analyzed the effect of three habitat and two disturbance covariates on Baird’s tapir occupancy. The first habitat covariate was the presence of water in the ponds because water is an essential feature for tapirs (Pérez-Irineo and Santos-Moreno 2016; Falconi-Briones et al. 2022). For the second habitat covariate, we identified the vegetation type present at each camera station, obtained from the land use vegetation map series VII 1:250,000 (INEGI 2022), given that tapirs show a preference for different vegetation types (Reyna-Hurtado and Tanner 2005; Falconi-Briones et al. 2022). The third habitat covariate was the normalized differential vegetation index (NDVI) using SENTINEL 2 satellite imagery with 10 m resolution, using the formula NDVI = (NIR - RED) / (NIR + RED) in the raster calculator from Qgis v. 3. 3. 1 software (QGis 2023). We considered the NDVI as a proxy of primary productivity, which is associated with food availability and quality for herbivores (Mena et al. 2020; Pettorelli et al. 2011).
The disturbance covariates were the distance to paved highways / paved state roads / unpaved local roads, and distance to the polygon of human settlements (houses, schools, other constructions), both obtained from the shape file “Marco geoestadístico” (INEGI 2022). Both covariates have had important effects on Baird’s tapir and other tapir species at other sites (Martinez et al. 2020; Mena et al. 2020; Ferreguetti et al. 2017; Cove et al. 2014). We estimated the Euclidean distance from the camera trap station to the nearest road and nearest human settlement using the vector analysis tool “shortest line between objects” in Qgis software (QGis 2023). Finally, we calculated the sampling effort as the number of days camera traps were active with the package CamtrapR in R software (Niedballa et al. 2022). Prior previous research in the region suggested that camera traps should be active for at least 45 to 60 days in tropical forests to obtain records for occupancy modelling of ungulate species, so we wanted to evaluate if the effort influenced tapir’s detectability (de la Torre et al. 2018; Martínez Martínez 2023).
Occupancy modelling. To determine the occupancy and detection probabilities of Baird’s tapir we constructed detection histories, separating the 50 days of sampling into 5 periods of 10 days each, considering that tapirs take 10 to 12 days to traverse their home range (Jordan 2015; de la Torre et al. 2018). Then we standardized the numeric covariates to a z-distribution (MacKenzie et al. 2006) and tested for autocorrelation using the variance inflation factor (VIF), where we excluded variables with a VIF > 3, a threshold used to minimize collinearity (Mena et al. 2020; Zuur et al. 2010).
Afterwards, we used a single season occupancy model, where we first built a null model, maintaining occupancy and detection constant, then a global model, which included all covariates to test the goodness of fit, and evaluated whether our data had a good fit to the less-complex models (MacKenzie and Bailey 2004). We calculated the value of the overdispersion factor (), which determined whether data had under-dispersion ( < 1), good fit (= 1), or over-dispersion ( > 1), with 10,000 bootstrap replications. If models presented over-dispersion, we used the Quasi-Akaike Criterion information (QAIC); otherwise, we used the conventional AIC (MacKenzie and Bailey 2004).
Subsequently, we evaluated effort as a covariate of detection probability, maintaining occupancy constant, and then we ranked it with the null model to test whether the sampling effort had a significant effect on detection probability. We ranked our models based on ΔAIC, where models with a ΔAIC < 2 were considered the best (Burnham and Anderson 2002). To determine whether the covariates from the best models had a significant effect on detection and occupancy, we considered the summed Akaike weights (Wall > 50 %) of the models and the p-value (p < 0.05) (Mena et al. 2020; de la Torre et al. 2018; Martinez et al. 2020). After determining whether the effort was an important covariate in the detection probability of Baird’s tapir, we built five occupancy models using a single covariate in each model, and then we ranked them and tested their importance for occupancy following the steps described previously. All the occupancy models used the unmarked function in R software v. 4. 2. 2 (Fiske and Chandler 2011).
Results
We registered 60 independent records of Baird’s tapir at 27 out of 36 ponds with a sampling effort of 1,599 camera trap nights. For occupancy modelling, the global model showed a good fit to the data (= 0.88, X2 = 25.3, p = 0.64).
For Baird’s tapir detection, the effort had a positive effect (β = 0.34 + 0.19, Wi = 0.66, p = 0.08; Figure 2) with a mean detection probability of 0.42 + 0.05, so we included this covariate for the next analyses (Table 1). We fitted six candidate occupancy models for Baird’s tapir records, including the null model (Table 1), where three models tested, including the null model, had a ΔAIC < 2, meaning they were moderately supported (Wall = 0.72). Distance to roads was the best-supported covariate, which had a positive effect on Baird’s tapir occupancy (β = 1.2 + 1.27, Wi = 0.39, p = 0.34; Figure 3). The mean distance of ponds to the different roads was 2.2 km, with a minimum distance to roads of 41 m and a maximum distance of 9.7 km. Distance to human settlements was the second-best ranked model with a positive effect (β = 0.49 + 0.65, Wi = 0.17, p = 0.45; Figure 4) on tapir occupancy. The mean distance of ponds to human settlements was 6.9 km, while the minimum distance was 653 m, and the maximum distance to human settlements was 24.6 km. Considering both covariates, the mean occupancy probability for Baird’s tapir was 0.85 + 0.11.
Discussion
Our findings supported our hypothesis that in communal lands from the Calakmul region, Baird’s tapirs are using ponds far from roads and human settlements with higher frequency than ponds close to roads and human settlements. This contrasts with what was reported in Belize, where tapirs used ponds near roads (Martinez et al. 2020). However, those roads in Belize were unpaved and with only light vehicle traffic. Paved roads unlike paved with heavy vehicle traffic that are dangerous for tapirs (Poot and Clevenger 2018; Contreras Moreno et al. 2013). Additionally, roads facilitate access to humans for hunting, logging, or other activities that may pose a threat to tapirs and other animals (Clements et al. 2014).
In a protected area in Peru, mountain tapirs use habitat away from roads to avoid potential threats like hunting (Mena et al. 2020). In a protected area in Brazil, lowland tapirs avoided areas with high poaching pressure and near roads (Ferreguetti et al. 2017). In southeast Asia, the Malayan tapir (Tapirus indicus) is affected by roads that cut their preferred habitat and where poaching is a potential threat (Clements et al. 2014).
As documented in several studies, all tapir species might be affected by roads due to their conditions and traffic, or when they are combined with other potential threats. In the Calakmul region, Baird’s tapirs are seldom hunted (Reyna-Hurtado and Tanner 2005) but there is a growing conflict because of damage to crops and the use of water from apiaries when seeking water by tapirs which may lead people to retaliate against them (Reyna-Hurtado and Tanner 2005; Pérez-Flores et al. 2021).
In our study site, some ponds were near highway México 186—a paved road with 1,823 vehicles moving daily in the Conhuas portion and 2,710 vehicles daily near Xpujil, according to the Ministry of Communications and Transport (SCT 2022); other ponds were near the state road CAM 269 where there are no records of the number of vehicles transiting, but the communities use this road to reach Xpujil, the main town in this area. The other paved road is the one to the archaeological site of Calakmul, with less traffic, used mainly by local people and tourists. The rest of the ponds were near local unpaved roads inside the communities, used only by local people. These roads have different conditions (paved and unpaved) and transit levels, but we did not have sufficient data to evaluate the impact of the different road types.
The distance to human settlements was the other covariate that best explained tapir occupancy. Martinez et al. (2020) also found in Belize that tapirs avoided ponds near villages. This avoidance of human settlements was also reported in Costa Rica in the Cordillera of Talamanca (Tobler 2002) and in San Juan - La Selva Biological Corridor (Cove et al. 2014). Although tapirs are not hunted in these sites either, other factors associated with villages--like dogs, deforestation, and hunting in the past--make the tapirs avoid these areas (Tobler 2002; Zapata-Ríos and Branch 2016). But, in specific conditions, tapirs may tolerate human presence to a certain degree. We observed a group of 3 tapirs in a pond (km 17) adjacent to the road to the archaeological site in Calakmul in the months of drought, when water is scarce in the reserve. But when the rainy season comes and water is available in the landscape, they move away from that pond, to avoid being near roads, as our study suggests (authors pers. obs).
In areas where Baird’s tapirs have suitable habitat, they will prefer to avoid contact with humans even if they are not hunted (Tobler 2002; Martinez et al. 2020; de la Torre et al. 2018; Cove et al. 2014); but in sub-optimal habitat conditions, they may tolerate a certain degree of disturbance (Reyna-Hurtado and Tanner 2005; 2007; Carrillo et al. 2019). In some places, tapirs would use towns to obtain water when drought is intense (Pérez-Flores et al. 2021) or to avoid potential predators (Pérez-Flores et al. 2022), although this might be an ecological trap if people from the communities retaliate against tapir because of any damage on crops (Medici et al. 2022).
In the Calakmul region, we find forested patches with suitable habitat for Baird’s tapirs in the three communities (Carrillo et al. 2019), especially because they have more ponds than in the CBR (Sánchez-Pinzón et al. 2020), but we also find potential threats like roads and human settlements that may reduce pond use (de la Torre et al. 2018; Cove et al. 2014; Carrillo-Reyna et al. 2015). Nonetheless, communal lands may be valuable for tapir conservation if they can be designated as community-protected areas. For example, in Nuevo Becal, a government program called Areas Voluntarily Designated for Conservation (ADVC for its Spanish initials) allows local farmers to extract timber and use different natural resources (Peña-Azcona et al. 2022). They also have legal hunting permits, which are carry out in Wildlife Conservation Management Units (UMA, by its acronym in Spanish). The UMAs are created and approved by the Ministry of Environment and Natural Resources (SEMARNAT, by its acronym in Spanish) so that specific wildlife populations are hunted with regulations (Gallina-Tessaro et al. 2009). These government programs improve the preservation of the remaining forests and wildlife populations, while helping residents from the communities to obtain economic benefits from those activities (Peña-Azcona et al. 2022). ADVC and UMAs might be useful tools for wildlife conservation outside federal or state reserves. Also, environmental education from academic institutions about the importance of ponds for tapirs and other wildlife (Martinez et al. 2020) can also encourage the people from the communities to protect the ponds by keeping them clean, avoiding deforesting near the ponds and loud noises so as not to scare away wildlife species.
Six months after our study ended, one of the biggest projects in southeast México began its construction, the Maya train, a project that has increased the intensity of use of both the highway and the state road evaluated near CBR. These changes could affect tapirs and other wildlife species. Since the economic development of the Calakmul region will likely increase in the next few years, the roads will probably get busier and the villages larger, which might represent a major threat to the connectivity of an already fragmented landscape (Carrillo et al. 2019). For this reason, it is of great importance that the wildlife passages that are being built allow the free movement of tapirs and other species in their habitat, so they can cross the railways and roads safely. It is also necessary to have strict surveillance of vehicle speed on the highways with speed cameras and patrols (Poot and Clevenger 2018).
Finally, even with our sample size, we obtained a relatively high detection probability for the time surveyed in comparison to other studies (Martinez et al. 2020; de la Torre et al. 2018). This is probably associated with the study design, since we evaluated ponds that may act as lures for tapirs (Martinez et al. 2020). However, we did not evaluate other habitat resources like refuge, understory cover, and food availability for tapirs in these communal lands, which might affect tapirs’ pond use. Therefore, we recommend evaluating other habitat features as well as the impacts of different types of roads, noise, the presence of feral dogs and cattle, and the distance to crops and railways outside the protected area. We also recommend increasing the sample size and effort to monitor ponds and other habitat features in all seasons of the year to reduce the potential bias the design might have on the results.
Acknowledgements
Special thanks to the Comisión Nacional de Áreas Naturales Protegidas (Project ProRest # Monitoreo de felinos silvestres y sus presas y otra fauna asociada en la Reserva de la Biosfera Calakmul - PROREST/ETM/29/2022) for the financial support in this project. Also, we thank Consejo Nacional de Humanidades, Ciencias y Tecnologías for the scholarship (884873) provided to JO-HR to study his PhD at El Colegio de la Frontera Sur. And we thank our local guides N. Arias Domínguez, R. Pech, M R Bacab Noh, E. Domínguez Bonilla, and A. Barrientos Ramírez for their support in the field.
Literature cited
Burnham, K.P., and D.R. Anderson. 2002. Model selection and multimodel inference: a practical information-theoretic approach (2nd ed). ecological modelling. Springer 172. New York, U.S.A.
Calderón-Aguilera, L. E., et al. 2012. An assessment of natural and human disturbance effects on Mexican Ecosystems: current trends and research gaps. Biodiversity and Conservation 21:589-617.
Carrillo, N., et al. 2019. Measuring landscape connectivity for Baird’s Tapir conservation in fragmented areas of Calakmul, Mexico. Tropical Conservation Science 12:1-15.
Carrillo, N., H. Weissenberger, and R. Reyna-Hurtado. 2015. Potential distribution of the Central American Tapir in the Yucatan Peninsula. Therya 6:575–96.
Carrillo-Reyna, N., R. Reyna-Hurtado, and B. Schmook. 2015. Abundancia relativa y selección de hábitat de Tapirus Bairdii en las reservas de Calakmul y Balam Kú, Campeche, México. Revista Mexicana de Biodiversidad 86:202–7.
Clements, G. R., et al. 2014. Where and how are roads endangering mammals in Southeast Asia’s forests. Plos One 9:1-25.
Contreras Moreno, F. M. et al. 2013. Nuevo registro de Tapir Centroamericano (Tapirus bairdii) atropellado en el noroeste del estado de Campeche, México. The Newsletter of the IUCN/SSC Tapir Specialist Group 22:22–25.
Cove, M. V., et al. 2014. Factors influencing the occurrence of the endangered Baird’s Tapir Tapirus bairdii: potential flagship species for a Costa Rican biological corridor. Oryx. 48:402-409.
de la Torre, J. A., et al. 2018 . Assessing occupancy and habitat connectivity for Baird’s Tapir to establish conservation priorities in the Sierra Madre de Chiapas, Mexico. Journal for Nature Conservation 41:16–25.
Doser, J. W., et al. 2022. SpOccupancy: an R package for single-species, multi-species, and integrated spatial occupancy models. Methods in Ecology and Evolution 13:1670–1678.
Estrada-Medina, H., et al. 2016. La Sequía de La Península de Yucatán. Tecnología y Ciencias Del Agua 3:151–65.
Falconi-Briones, F. A., et al. 2022. Habitat use and activity patterns of ungulates in a tropical rainforest of Southern México. Therya 13:171–82.
Ferreguetti, A. C., W. M. Tomas, and H. G. Bergallo. 2017. Density, occupancy, and detectability of Lowland Tapirs, Tapirus Terrestris, in Vale Natural Reserve, Southeastern Brazil. Journal of Mammalogy 98:114–23.
Fiske, I. J, and B. Chandler. 2011. Unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. JSS Journal of Statistical Software 43.
Gallina-Tessaro S. A. et al. 2009. Unidades para la conservación, manejo y aprovechamiento sustentable de la vida silvestre en México (UMA). Retos para su correcto funcionamiento. Investigación ambiental 1:43–152.
García, M. et al. 2016. Tapirus Bairdii. The IUCN Red List of Threatened Species 2016. www.iucnredlist.org. Accessed on 1 June 2024.
INE. 2000. Programa de manejo de la Reserva de la Biósfera Calakmul. Edited by Julia Carabias Lillo, Enrique Provencio, Javier de la Maza Elvira, and José Bernardo Rodríguez de la Gala Méndez. 1st ed. Vol. 1. Mexico City: INE.
INEGI. 2022. Sistema de Consulta-Mapas. 2022. https://www.inegi.org.mx/app/mapas/.
Jordan, C. A. 2015. The dynamics of wildlife and environmental knowledge in a bioculturally diverse coupled natural and human system in the Caribbean region of Nicaragua. Doctor of Philosophy, Michigan: Michigan State University.
MacKenzie, D. I., and L. L. Bailey. 2004. Assessing the fit of site-occupancy models. Journal of Agricultural, Biological, and Environmental Statistics 9:300–318.
MacKenzie, D. I., et al. 2002. Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–55.
MacKenzie, D. I., et al. 2006. Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. Elsevier I. Vol. 1. San Diego, California.
Martínez Martínez, L. V. 2023. Ocupación y tasa de visita de ungulados en un área de conservación voluntaria en Calakmul, Campeche. Master degree, El Colegio de la Frontera Sur.
Martinez, W. E., R. A. Reyna-Hurtado, E. J. Naranjo, D. Thornton, R. N. Cal, and O A. Figueroa. 2020. Occupancy rate and observations of Baird’s Tapir (Tapirella Bairdii) near waterholes in the Maya Forest Corridor, Belize. Therya 11:1–7.
Medici, E. P., et al. 2022. Movement ecology of vulnerable lowland tapirs between areas of varying human disturbance. Movement Ecology 10:1–12.
Mena, J. L., H. Yagui, F. La Rosa, P. Pastor, J. Rivero, and R. Appleton. 2020. Topography and disturbance explain Mountain Tapir (Tapirus Pinchaque) occupancy at its southernmost global range. Mammalian Biology 100:231–39.
Meyer, N. F. V., R. Moreno, R. Reyna-Hurtado, J. Signer, and N. Balkenhol . 2020. Erratum: Towards the restoration of the Mesoamerican Biological Corridor for large mammals in Panama: comparing multi-species occupancy to movement models. Movement Ecology 8:1–14.
Naranjo, E. J. 2018. Baird’s Tapir ecology and conservation in Mexico revisited. Tropical Conservation Science 11:1-5.
Naranjo, E. 2019. Tapirs of the Neotropics. Pp. 39–51 in ecology and conservation of tropical ungulates in Latin America, Springer International Publishing.
Niedballa, J, A. Courtiol, and R. Sollman. 2022. Package CamtrapR. R software.
O’Farrill, G., et al. 2012. Effective dispersal of large seeds by Baird’s Tapir: a large-scale field experiment. Journal of Tropical Ecology 28:119–22.
O’Farrill, G., M. Galetti, and A. Campos-Arceiz. 2013. Frugivory and seed dispersal by tapirs: an insight on their ecological role. Integrative Zoology 8:4–17.
O’Farrill, G., et al. 2014. The potential connectivity of waterhole networks and the effectiveness of a protected area under various drought scenarios. Plos One 9:1-10
Pennington, T. D., and J. SarukhánJ. 2005. Árboles tropicales de México: manual para la identificación de las principales especies. UNAM.
Peña-Azcona, I, A., et al. 2 022. Áreas de Conservación Voluntaria en México: alcances y desafíos. Revista de Ciencias Ambientales 56:122–47.
Pérez-Cortez, S., et al. 2012. influencia de la disponibilidad de agua en la presencia y abundancia de Tapirus Bairdii en la selva de Calakmul, Campeche, México. Revista Mexicana de Biodiversidad 83:753–61.
Pérez-Flores, J., et al. 2021. Human-wildlife conflicts and drought in the greater Calakmul Region, Mexico: implications for tapir conservation. Neotropical Biology and Conservation 16:539–63.
Pérez-Flores, J., et al. 2022. Jaguar’s predation and human shield, a tapir story. Diversity 14:1-12.
Pérez-Irineo, G., and A. Santos-Moreno. 2016. Abundance, herd size, activity pattern and occupancy of ungulates in Southeastern Mexico. Animal Biology 66:97–109.
Pettorelli, N., et al. 2011. The Normalized Difference Vegetation Index (NDVI): unforeseen successes in animal ecology. Climate Research 46:15-27.
Poot, C., and A. P. Clevenger. 2018. Reducing vehicle collisions with the Central American Tapir in Central Belize District, Belize. Tropical Conservation Science 11:1–7.
Pukazhenthi, B., et al. 2013. A review of the reproductive biology and breeding management of tapirs. Integrative Zoology 8:18-34.
QGis. 2023. “QGis 64bit.” Boston, MA: Free Software Foundation.
Reyna Hurtado, R. A et al. 2022. Aguadas de la Selva Maya: santuarios de vida silvestre que unen esfuerzos de conservación internacional. Ciencia Nicolaita 84:72-80.
Reyna-Hurtado, R., and G. W. Tanner. 2005. Habitat preferences of ungulates in hunted and nonhunted areas in the Calakmul. Biotropica 37:676–685.
Reyna-Hurtado, R., and N. Arias Domínguez. 2024. Baird’s Tapir social interactions, activity patterns, and site fidelity at ponds of the Maya Forest. Therya 15:29–37.
Reyna-Hurtado, R., et al. 2016. Insights into the multiannual home range of a Baird’s Tapir (Tapirus Bairdii) in the Maya Forest. Therya 7:271–76.
Reyna-Hurtado et al. 2019. Tapir Population patterns under the disappearance of freestanding water. Therya 10:353–58.
Reyna-Hurtado, R., and G. W. Tanner. 2007. Ungulate relative abundance in hunted and non-hunted sites in Calakmul Forest (Southern Mexico). Biodiversity and Conservation 16:743–56.
Rivero, M., et al. 2022. Tapirs in trouble: estimating Baird’s Tapir densities in the Sierra Madre de Chiapas, Mexico. Oryx 56:373–82.
Sánchez-Pinzón, K., R. Reyna-Hurtado, and N. F. V. Meyer. 2020. Moon light and the activity patterns of Baird’s Tapir in the Calakmul Region, Southern Mexico. Therya 11:137–42.
Sandoval Serés, E., et al. 2016. Uso de aguadas y abundancia relativa de tapirus bairdii en la región de Calakmul, Campeche, México. Therya 7:39–50.
Schank, C. J. et al. 2020. population status, connectivity, and conservation action for the endangered Baird’s Tapir. Biological Conservation 245:1-12.
Schank, C. J. et al. 2017. Using a novel model approach to assess the distribution and conservation status of the endangered Baird’s Tapir. Diversity and Distributions 23:1459–1471.
SCT. 2022. Datos viales de Campeche. Secretaría de Caminos y Transportes. www.Sct.Gob.Mx/Fileadmin/DireccionesGrales/DGST/Datos_Viales_2022/04_CAMP_DV2022.Pdf. Accessed on 20 August 2024.
Serrano-Mac-Gregor, I., et al. 2021. Baird’s Tapir: predicting patterns of crop damage surrounding the Calakmul Biosphere Reserve, Campeche, Mexico. Revista Mexicana de Biodiversidad 92:1-14.
Tobler, M. W. 2002. Habitat use and diet of Baird’s Tapirs (Tapirus Bairdii) in a montane cloud forest of the Cordillera de Talamanca, Costa Rica. Biotropica 34:468–74.
Trepel, J., et al. 2024. Meta-analysis shows that wild large herbivores shape ecosystem properties and promote spatial heterogeneity. Nature Ecology and Evolution 1-21.
Zapata-ríos, G., and L. C Branch. 2016. Altered activity patterns and reduced abundance of native mammals in sites with feral dogs in the High Andes. Biological Conservation 193:9–16.
Zuur, A. F., E. N. Ieno, and C. S. Elphick. 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1:3–14.
Associated editor: Alina Gabriela Monroy-Gamboa
Submitted: June 20, 2024; Reviewed: June 28, 2024
Accepted: September 10, 2024; Published on line: September 17, 2024

Figure 1. Camera trap survey design in ponds from the communities of Zoh Laguna, Conhuas, and Nuevo Becal, Campeche, México.
Table 1. Occupancy models selected according to AIC for Baird’s tapir.
|
Common name |
Species |
Detection Model |
k |
AIC |
ΔAIC |
Wi |
Wall |
|
Baird’s tapir |
Tapirus bairdii |
p(Effort) Ψ(.) |
3 |
225.26 |
0 |
0.66 |
0.66 |
|
p(.)Ψ (.) |
2 |
226.61 |
1.35 |
0.34 |
1 |
||
|
Occupancy Model |
k |
AIC |
ΔAIC |
Wi |
Wall |
||
|
p(Effort) Ψ (DR) |
4 |
224.83 |
0 |
0.39 |
0.39 |
||
|
p(Effort) Ψ (DL) |
4 |
226.50 |
1.67 |
0.17 |
0.56 |
||
|
p(.)Ψ (.) |
2 |
226.61 |
1.79 |
0.16 |
0.72 |
||
|
p(Effort) Ψ (Water) |
4 |
226.86 |
2.03 |
0.14 |
0.86 |
||
|
p(Effort) Ψ (NDVI) |
4 |
227.18 |
2.36 |
0.12 |
0.98 |
||
|
p(Effort) Ψ (Veg) |
7 |
230.69 |
5.86 |
0.02 |
1 |
Note: Effort=Number of days cameras were active; DR= Distance to roads; DL= Distance to human settlements; Water=Presence of water in ponds; NDVI=Normalized difference vegetation index; Veg=Vegetation type; K= Number of parameters; AIC= Akaike Criterion Information; ΔAIC= Delta Akaike Criterion Information; Wi= Weighted Akaike Criterion Information; Wall= Accumulated weighted Akaike Criterion Information.
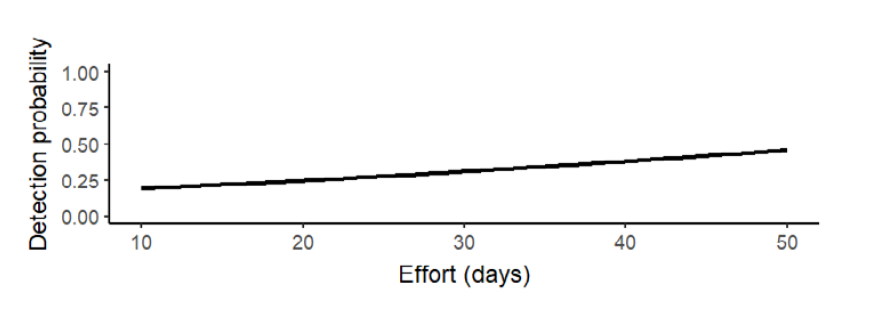
Figure 2. Effect of sampling effort (number of days camera traps were active) on Baird’s tapir detection probability in ponds on communal lands.
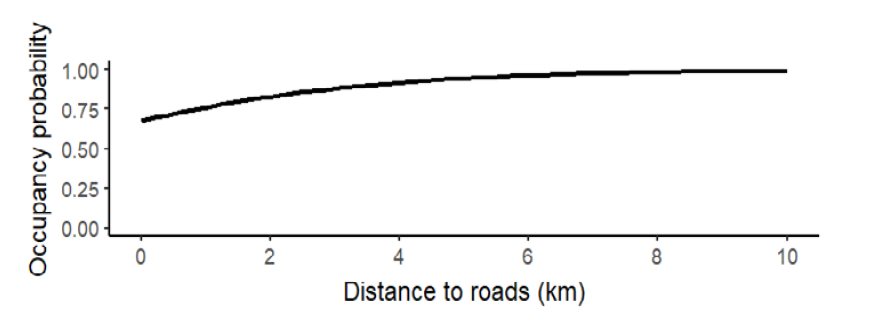
Figure 3. Effect of distance to roads (km) on occupancy probability of Baird’s tapir in ponds on communal lands.
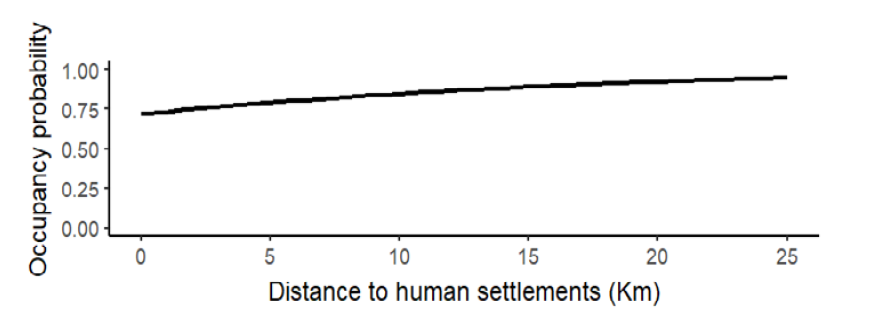
Figure 4. Effect of distance to human settlements (km) on occupancy probability of Baird’s tapir in ponds on communal lands.