THERYA, 2024, Vol. 15(3):289-301 DOI:10.12933/therya-24-6140 ISSN 2007-3364
Potential distribution of the Curaçaoan Long-nosed Bat, Leptonycteris curasoae: implications for monitoring and conservation
Aída Otálora-Ardila1, Ángela P. Cuervo-Robayo2*, Jafet M. Nassar3, María Camila Valdés-Cardona1, Camila A. Díaz-B1,
Maria P. Henáo-Rodríguez1, Hugo F. López-Arévalo1, and Olga L. Montenegro1
1 Universidad Nacional de Colombia, Sede Bogotá, Facultad de Ciencias, Instituto de Ciencias Naturales, Grupo en Conservación y Manejo de Vida Silvestre. E-mail: aotalora@gmail.com (AO-A); mcvaldesc@unal.edu.co (MCV-C), cadiazbe@unal.edu.co (CAD-B), mphenaor@unal.edu.co (MPH-R), hflopeza@unal.edu.co (HFL-A), olmontenegrod@unal.edu.co (OLM).
2 Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México, Ciudad de México, México. E-mail: ancuervo@gmail.com (APC-R).
3 Centro de Ecología, Instituto Venezolano de Investigaciones Científicas, Caracas, Venezuela. E-mail: jafet.nassar@gmail.com (JMN).
* Corresponding author https://orcid.org/0000-0002-8490-4095.
Understanding the spatial distribution of animal species, which involves integrating species occurrence with environmental data, provides crucial information for conservation planning, especially for threatened species. In this study, we used niche-based species distribution models to create a potential distribution map of Leptonycteris curasoae, a vulnerable fruit- and nectar-feeding bat. This model incorporated occurrence data from field sampling, mammal collections, scientific literature, and environmental variables. Additionally, we mapped the threats faced by L. curasoae and overlaid this data with protected area boundaries to identify priority conservation regions. Our results indicate that the current and potential distribution of this species is considerably smaller (~9 %) than the area previously considered according to the IUCN Red List. The potential distribution of L. curasoae is environmentally restricted to arid and semiarid areas and dry forests in Aruba, Bonaire, Curaçao, Northern Colombia, and Venezuela, characterized by high temperatures, low precipitation, and seasonality in temperature and precipitation. Approximately 22 % of the suitable areas for this species are within protected areas, and we observe differences in the magnitude of the area under protection and the impact of identified threats among countries. Roost vandalism is the most critical threat in Curaçao and Venezuela, while mining, tourism, and wind farms are more frequent in Colombia. Expanding or creating protected areas and roosts, jointly with establishing conservation corridors and connected private reserves across political boundaries, are high-priority conservation actions needed to guarantee safe mating and maternity roosts, long-distance movements, and connectivity of L. curasoae populations along its entire geographic distribution.
Comprender la distribución espacial de las especies animales que involucran conjuntamente la presencia de especies y los datos ambientales ofrece información crítica para la planificación de la conservación, particularmente para las especies amenazadas. Aquí, utilizamos modelos de nicho para estimar la distribución potencial de Leptonycteris curasoae, un murciélago frugívoro-nectarívoro considerado como Vulnerable. Este modelo incluyó datos de presencia provenientes de muestreos en campo, colecciones de mamíferos, literatura científica, así como variables ambientales. Adicionalmente, mapeamos las amenazas actuales para superponerlas con datos de áreas protegidas y así poder identificar áreas prioritarias para conservación de L. curasoae. Nuestros resultados indican que la distribución actual y potencial de esta especie es menor (9 %) que la considerada anteriormente en la Lista Roja de Especies Amenazadas de la UICN. La distribución potencial de L. curasoae está ambientalmente restringida a áreas áridas, semiáridas y bosques secos en Aruba, Bonaire, Curazao, el norte de Colombia y Venezuela, caracterizados por altas temperaturas, bajas precipitaciones y estacionalidad en temperatura y precipitación. Aproximadamente el 22 % de las áreas adecuadas para esta especie está dentro de áreas protegidas, y observamos diferencias en la magnitud de área protegida y el impacto de las amenazas identificadas entre países. El vandalismo es la amenaza más crítica en Curazao y Venezuela, mientras que la minería, el turismo y los parques eólicos son más frecuentes en Colombia. Ampliar o crear áreas y refugios protegidos, junto con el establecimiento de corredores de conservación y reservas privadas conectadas más allá de las fronteras políticas, son acciones de conservación de alta prioridad para garantizar la permanencia de refugios de apareamiento y maternidad, los movimientos de larga distancia y la conectividad de las poblaciones de L. curasoae a lo largo de su distribución.
Keywords: Chiroptera; Curaçaoan Long-nosed Bats; species distribution models; tropical dry forest; vulnerable bat species.
© 2024 Asociación Mexicana de Mastozoología, www.mastozoologiamexicana.org
Introduction
Global trends indicate that human activities have significantly impacted biodiversity, particularly in the past five decades (Díaz et al. 2019). Currently, mammals are the second most endangered vertebrate group, comprising 22 % of threatened species (IUCN 2020). Among mammals, 15 % of bat species are threatened (Frick et al. 2020). Although bats have a global distribution and high diversity in tropical regions (Altringham 2011; Burgin et al. 2018), many species are sensitive to habitat disturbances (Voigt and Kingston 2016).
The Curaçaoan Long-nosed bat, Leptonycteris curasoae Miller, 1900, is one of the 19 threatened bat species in South America and the Caribbean islands. This species strongly depends on dry forests and arid ecosystems in Colombia, Venezuela, and the Caribbean islands (IUCN 2023). This fruit- and nectar-feeding bat species is the primary pollinator of several plant species, mainly of Cactaceae (e. g., Stenocereus spp., Cereus spp., Pilosocereus spp.) and Asparagaceae (Agave spp.) families (Griffiths and Gardner 2007). L. curasoae is a migratory bat, undertaking long-distance flights (28 to 89 km) between Caribbean islands, including Aruba, Bonaire, and Curaçao, and between those islands and the mainland of northern Venezuela (Simal et al. 2015).
The main threats for L. curasoae are habitat alteration due to urban expansion, mining, quarrying, wind farms development, and vandalism of roosts, mostly caves (Nassar 2015; Simal et al. 2021). Furthermore, dry forests, arid and semiarid zones are the most threatened and transformed ecosystems in the Neotropics (Escribano-Avila et al. 2017), with 66% of the dry forests having already disappeared (Portillo-Quintero and Sánchez-Azofeifa 2010). In Colombia, Venezuela, and the Caribbean islands, these ecosystems are in critical condition because they are underrepresented in protected areas. In Colombia and Venezuela, only 5 % of their coverage is under protection (Fajardo et al. 2005; Rodríguez et al. 2010; Pizano and García 2014), and in Caribbean islands, approximately 10 % of these ecosystems are within protected areas (Portillo-Quintero and Sánchez-Azofeifa 2010).
Most studies assessing habitat and feeding ecology, geographic range, roost location, and populational trends of L. curasoae have been carried out in the islands of Aruba, Bonaire, and Curaçao (Petit 1995, 1997; Simal et al. 2015, 2021) and Venezuela (Martino et al. 1998, 2002; Nassar et al. 2003; Newton et al. 2003). Much less information is available for Colombia (Cadena et al. 1998; Sánchez and Cadena 1999). The above topics are vital for updating the risk of extinction assessment provided by the IUCN Red List nearly a decade ago for L. curasoae (Nassar 2015). Information regarding long-distance routes, stopover foraging grounds, and diurnal and mating/maternity roosts along its potential seasonal migration paths remains scarce across L. curasoae´s entire geographical distribution, limiting our understanding of the annual migration dynamics attributed to this species.
Niche-based species distribution modeling (SDM) has proved to be valuable in conservation strategies (Rodríguez et al. 2007; Villero et al. 2017). Such modeling is particularly useful for locating suitable areas for field exploration and identifying key variables limiting the distribution of rare bat species of conservation concern (Razgour et al. 2011). In this study, we aimed 1) to generate a potential distribution map of L. curasoae, 2) to identify threats and under-protected areas within this bat's potential distribution, and 3) to fill in information gaps concerning the current distribution and roosts occurrence for the species, emphasizing understudied countries such as Colombia.
Materials and methods
Study area. We compiled data from all countries where L. curasoae is distributed: Colombia, Venezuela, Aruba, Bonaire, and Curaçao islands (ABC islands). Due to the limited information available on the current distribution of L. curasoae in Colombia, we conducted field expeditions in 2020, 2022, and 2023 according to the reproductive cycle and the possible annual migration of the species (Martino et al. 1998; Simal et al. 2021). Field expeditions were conducted in 27 locations across three geographic areas: 1) The Chicamocha River basin (ten sites) in January 2020. 2) the Caribbean coast (nine sites) in February 2020. 3) Tierra Bomba island in February 2020, April and December 2022 and June 2023. 4) La Guajira Peninsula (seven sites, Supplementary material Figure S1) in April 2022.
The Chicamocha River Basin is in the Colombian Eastern Mountain range, at 500 to 1,500 masl (Albesiano et al. 2003). This area features semi-arid, thorn scrub, and dry forest vegetation (Latorre et al. 2014). The mean annual temperature in this region ranges from 16 ºC to 36 ºC, and annual rainfall reaches 731 mm (Albesiano et al. 2003). Sampled localities in the Caribbean coast and La Guajira Peninsula belong to the Peri-Caribbean Arid Belt. The Caribbean coast is characterized by seasonally tropical dry forests, with an average annual temperature of 27 °C (16 to 36 °C, IDEAM 2010), while La Guajira Peninsula primarily comprises xerophytic and thorn scrub vegetation, with an average annual temperature of 29 °C (19 to 36 °C, IDEAM 2010). The Peri-Caribbean Arid Belt province generally has lower average yearly rainfall, strong winds, and higher evaporation rates than the Chicamocha River Basin (Hernández et al. 1992).
Current geographic distribution of Leptonycteris curasoae. We mapped the current geographical distribution of L. curasoae revisiting information on specimens deposited in mammal collections, including the Instituto de Ciencias Naturales of Universidad Nacional de Colombia (ICN), Instituto Alexander von Humboldt (IAvH), Universidad Industrial de Santander (UIS-MHN), and Vertebrate collection at Universidad de Los Andes, Venezuela (CVULA). Additional records were obtained from other institutions by revising databases such as GBIF (GBIF 2022) and VertNet (VertNet 2022). Furthermore, we examined information about geographical distribution in the scientific literature in Google Scholar (Google Scholar 2022) and Web of Science (Web of Science 2022) using the keywords “Leptonycteris* AND L. curasoae* AND Curaçaoan Long-nosed Bat*” without date restrictions. Also, we checked data compiled in the thesis and technical reports of studies carried out in zones where this bat species was historically found. This resulted in a total of 13 documents conducted in the five countries where L. curasoae is distributed. We assessed the Extent of Occurrence (EOO) and the Area of Occupancy (AOO) for the revisited current distribution that we obtained and for the previous distribution published in the last IUCN Red List assessment (Nassar 2015), using the sRedList platform (Cazalis et al. 2024).
As described earlier, we complemented the current distribution for Colombia conducting field expeditions across 27 locations. At each location, bat sampling was carried out using six ground-level mist nets (two 6 m, one 9 m, and three 12 m length, 20 mm mesh, ECOTONE, Poland). Mist nets were active from 18:00 to 00:00 and were checked every 25 minutes on average. Our total sampling effort was 16,609 m2 x mist net x hours (mnh). Also, we searched for caves and artificial roosts where bats were captured using mist nets at the entrances of the roosts or an entomological hand net inside the roosts. Capture and handling methods followed the guidelines of the American Society of Mammalogists (Sikes et al. 2011) and were authorized by licenses from the Environmental Licenses National Agency (ANLA 2014).
Ecological modeling and climate variables. We used 19 current bioclimatic variables sourced from the Chelsa database (Karger et al. 2021), which offers data at approximately 1 km² spatial resolution (0.0083 arc seconds). These variables depict annual and seasonal climate patterns over the baseline period from 1970 to 2000. To address collinearity issues among the 19 bioclimatic variables, we employed the corrSelect function from the fuzzySim package (Barbosa 2015), available in R (R Core Team 2022). By using the Pearson method with a threshold value set at 0.8 and a variance inflation factor, this function effectively identifies and excludes highly correlated variables (Barbosa 2015). To establish a calibration area (M), we intersected a buffer around species occurrences (2.5 °) with ecoregions defined by Olson et al. (2001). This approach considers both dispersal and ecological limitations, enhancing the robustness of our modeling process.
Occurrence records. We generated a database comprising 400 georeferenced records for L. curasoae and after a thorough examination, we excluded 63 % of the records due to inconsistent or duplicated coordinates. Furthermore, we implemented a distance-based filter of 5 km to mitigate sample bias, using the spThin function (Aiello-Lammens et al. 2015) of the Wallace package (Kass et al. 2018, 2023). As a result of these procedures, we obtained 148 valid records.
Niche-based species distribution model. We employed the ensemble modeling approach implemented in the biomod2 package (Thuiller et al. 2009) within the R software (R Core Team 2022) to develop the niche-based distribution model for L. curasoae. Ensemble modeling combines multiple individual models to produce a more robust prediction by leveraging the strengths of each constituent model while mitigating the effects of their inherent uncertainties and errors (Araujo and New 2007).
Out of the ten algorithms available in biomod2, we selected seven for our analysis: MaxEnt (MAXENT.Phillips), Generalized Additive Models (GAM), Generalized Boosted Regression Model (GBM), Artificial Neural Networks (ANN), Surface Range Envelope (SRE), and Random Forest (RF). Because our occurrence datasets only included presence-derived information, we randomly generated 10,000 pseudo-absences within the calibration area to balance the dataset. Prevalence was set to 0.5 to assign equal importance to presences and pseudo-absences during the calibration process. Each model was executed ten times, with each run using a different selection of calibration and evaluation datasets, with 70 % of the data allocated for calibration and the remaining 30 % for evaluation in each run.
To evaluate model performance, we used the area under the curve of the receiver operating characteristic (AUC). Models were assembled using a total consensus rule, where algorithms and the ten replicates were assembled based on the weighted sum of evaluations greater than AUC > 0.8 (Araujo and New 2007). Subsequently, an ensemble model comprising seven algorithms × 10 repetitions was used to project the potential distribution of L. curasoae under current climatic conditions. Given the thorough revision of all occurrence records (Peterson et al. 2011), we are confident that each point contributes to representing the distribution of L. curasoae. Therefore, we obtained a binary map by employing the minimum training presence threshold (value of 240).
Pressures and threats. We conducted an overlay analysis, superimposing the current and potential spatial distribution of L. curasoae with layers depicting land use transformation and protected areas, and mapped current threats such as mining, wind farms, tourism, and vandalism of roosts across the geographic range of this species. The land-use transformation data were obtained from a global map of land use/land cover (LULC) from 2023 derived from ESA Sentinel-2 imagery at 10 m resolution (Karra et al. 2021). This map layer was created based on a large dataset of over 5 billion human-labeled Sentinel-2 pixels by developing and deploying a deep-learning segmentation model on Sentinel-2 data. The algorithm generates LULC predictions for nine classes: water, trees, flooded vegetation, crops, built area, bare ground, snow/ice, clouds, and rangeland.
The polygons representing protected areas were sourced from Protected Planet (Protected Planet 2023), a comprehensive data repository on protected areas and other effective area-based conservation measures (OECMs). This database is updated monthly and maintained by the United Nations Environment Programme World Conservation Monitoring Centre (UNEP-WCMC 2024). Only protected areas categorized by the IUCN were considered. Protected areas designated as ‘Protective Zones’ and ‘Critical Areas with Treatment Priority’ were excluded from the analysis because they only exist in Venezuela, and the anthropogenic activities allowed include exploitation of natural resources incompatible with the aims of protected areas (García and Silva 2013). We compiled 340 georeferenced locations of mining, wind farms, and tourism threats obtained from Colombian governmental platforms such as the Mining and Energy Planning Unit (2023) and the Ministry of Commerce, Industry, and Tourism (2022), respectively. For Aruba, Bonaire, Curaçao, and Venezuela, threat information came from published data (Petit et al. 2006; Molinari et al. 2012; Nassar and Simal 2019; Simal et al. 2021) and data observed directly in the field between 1997 and 2015 (J. Nassar pers. obs.). Similarly, roost vandalism records for Colombia were obtained from our field observations from 2020 to 2023.
By integrating spatial data on land cover change, we identified regions where habitat degradation and fragmentation might pose significant threats to L. curasoae populations. Additionally, overlaying data of protected areas allowed us to assess the extent to which current conservation measures cover the bat's distribution range and identify potengial gaps in its protection. Mapping current threats enabled us to pinpoint specific areas where human activities pose immediate risks to the survival and habitat integrity of L. curasoae.
Results
Current geographic distribution of L. curasoae. Most of the occurrence records of L. curasoae came from Venezuela (41.2 %) and Colombia (39.9 %), and the remaining occurrence data (18.9 %) correspond to the ABC islands (Supplementary material, Table S1). In Colombia, we captured 200 individuals from six localities in the three geographic areas sampled: the Chicamocha River Basin (5 individuals), the Caribbean Coast (124), and La Guajira Peninsula (71; Table S2). Five localities represent new records of L. curasoae in the country (Table S1).
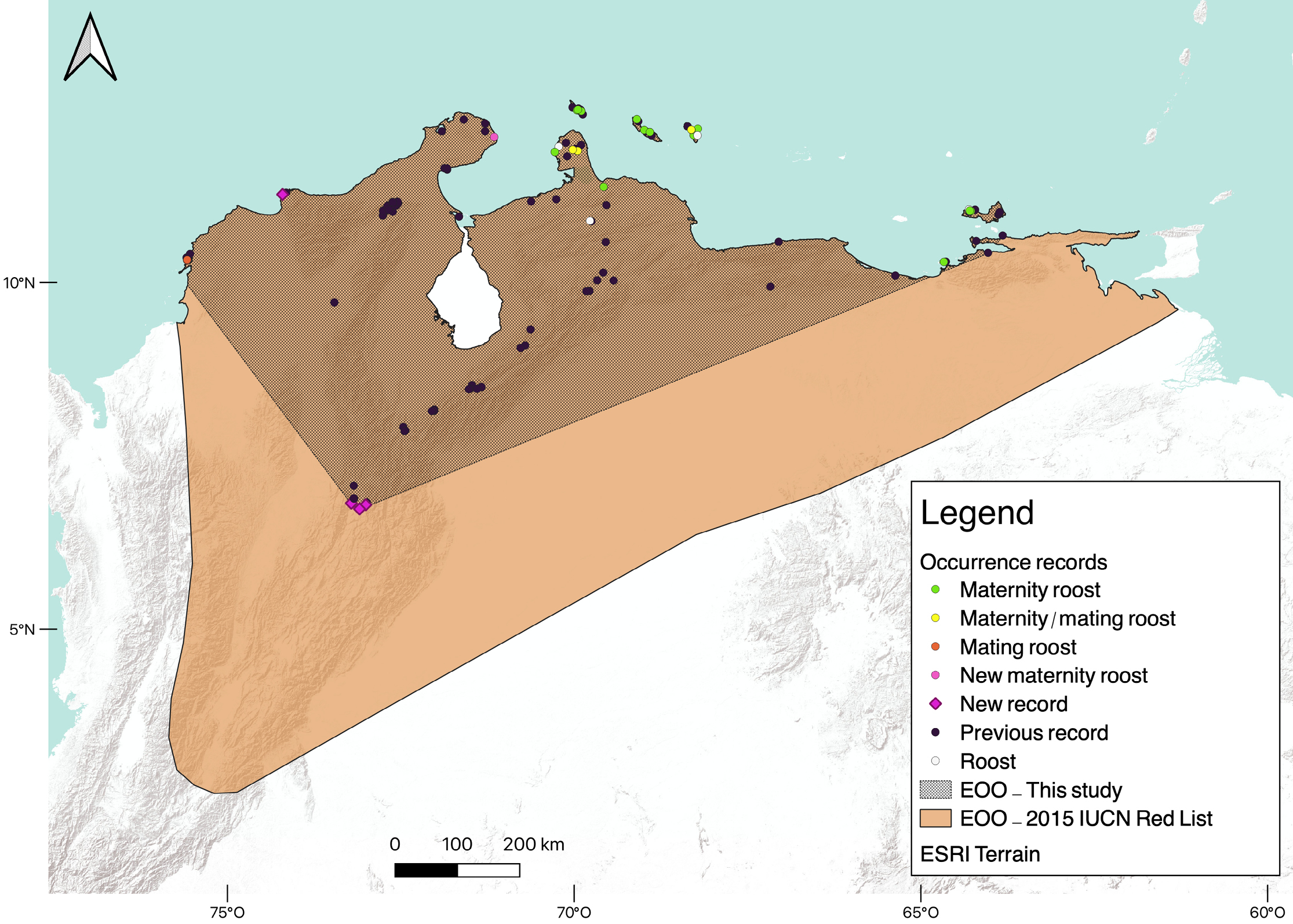
Throughout the geographic range of L. curasoae, only 23 locations (15.5 %) correspond to roosts. Ten roosts are in Venezuela, and ten are in the ABC islands. During the field expeditions in Colombia, we explored 23 roosts, including caves, natural shelters, and human-made buildings (Supplementary material, Table S3). We discovered three roosts of L. curasoae in Colombia. One roost is in a cave on La Guajira Peninsula, which hosts a large maternity colony (Table S3). The other two roosts are in historical fortresses on Tierra Bomba Island where a smaller colony resides. This colony was observed only in February 2020 and December 2022, using the fortresses as mating roosts (Table S3). Eighteen shelters are maternity roosts: Venezuela (7 shelters), Bonaire (4), Aruba (3), Curaçao (3), and Colombia (1). Mating roosts were found in Venezuela (5), Bonaire (2), and Colombia (2; Supplementary material, Table S1). Our data indicated that the Extent of Occurrence (453,300 km2) was reduced by almost half and the Area of Occupancy (167,500 km2) by nearly a third in the revisited current distribution compared to the previous distribution published in the last IUCN Red List assessment (EOO = 1,028,661km2 and AOO = 614,428 km2; Figure 1).
Niche-based species distribution model. Out of the 60 models created (six algorithms and ten replicates), 45 models exhibited AUC values greater than 0.8. The average sensitivity across these models was 96.5 %, indicating a high proportion of correctly predicted presences, while the average specificity was 89.4 %, reflecting a high proportion of correctly predicted absences (Supplementary material, Table S4).
The potential distribution of L. curasoae estimated through the weighted ensemble of these models encompassed most of its currently known distribution in Colombia, Venezuela, and the ABC Islands. Predicted suitable areas were predominantly identified in Aruba, Bonaire, Curaçao, La Guajira Peninsula in Colombia, the Paraguaná Peninsula, and areas south of Falcón and north of Lara states in Venezuela, as well as several intra-Andean arid patches in Venezuela and Colombia. All these areas correspond with most of the currently known locations for this species, however, some exceptions were observed. Suitable areas for L. curasoae were identified in the northeast portion of the Sierra de Perijá, north of Monagas state, and southeast of the Paraguaná Peninsula in Venezuela, as well as in the south of Sierra del Perijá and south of the Chicamocha River Basin in Colombia. All of them are locations where L. curasoae has never been recorded in the past. Additionally, a deviation of the model was detected with no suitable area predicted for an existent record of the species in the south of the Cesar state, in Colombia (Figure 2).
The final set of non-correlated variables was seven (Table 1). Based on the model’s predictions, L. curasoae can occur in areas with relatively high annual mean temperature, low temperature seasonality, and low variation in annual mean diurnal range temperature. Also, suitable areas are characterized by low to intermediate annual precipitation, intermediate precipitation seasonality, and low precipitation in the driest month and the coldest quarter (Table 1). The potential distribution model indicated that suitable areas for this bat species represent only 9.4 % of the total area reported in the IUCN Red List (Figure 2).
Pressures and threats. According to the LULC map, the most dominant land-use classes were rangeland (47.6 %) and trees (40.2 %). In comparison, the anthropized area covered 8.4 % of the potential distribution area of L. curasoae (Supplementary material, Table S5). In general, 133 protected areas overlapped with the potential distribution of L. curasoae (Figure 2, Supplementary material, Table S6). Only 40 (30.1 %) protected areas have the highest level of protection, being considered as IUCN categories Ia (1 area) and II (39), which cover only 18.41 % of the potential distribution of L. curasoae. Most of the protected areas are in Venezuela (58.6 %). In Colombia, 33.1 % of the protected areas overlapped, while in the ABC islands the overlap was 8.3 %. About 22.2 % of the suitable areas predicted are inside protected areas, most of which are in Venezuela (20.2 %), followed by Colombia (1.9 %) and the ABC islands (0.1 %; Table 2).
We identified 86 threats that overlapped with the potential distribution of L. curasoae (Figure 2). Roost vandalism is the most critical threat in Curaçao and Venezuela, while mining, tourism, and wind farms are the most frequent menaces identified in Colombia. Mining and tourism were mainly concentrated in L. curasoae suitable areas in the intra-Andean, Caribbean, and La Guajira regions of Colombia. Wind farms are mainly restricted to the ABC islands (1 wind farm in each) and northern Colombia (17; Figure 2, Supplementary material, Table S7).
Discussion
The distribution of a species is expected to occur in areas with suitable abiotic conditions and biotic interactions that have been accessible to the species via dispersal over relevant time periods (Feng et al. 2024). In this study, we found that the current Extent of Occupancy and potential distribution of L. curasoae are smaller than previously considered, with environmentally suitable areas found in the ABC islands, northern Colombia and Venezuela. Around 22.3 % of the suitable areas for the species are inside protected areas, and many threats identified occur in countries where the environmentally suitable areas are less represented in national parks or reserves, such as Colombia.
Current geographic distribution of L. curasoae. In Colombia, L. curasoae is more abundant in the coastal region (Caribbean and La Guajira Peninsula) than in the Chicamocha River Basin. Similarly, more occurrences of this species were reported by Cañón and Trujillo (2014) in La Guajira. Previous studies have documented a lower capture rate of L. curasoae in intra-Andean arid patches of Colombia and Venezuela compared to northern Venezuela (Sosa and Soriano 1993; Cadena et al. 1998; Martino et al. 1998; Sánchez and Cadena 1999; Soriano et al. 2000). Part of the sampling in the Caribbean coast and La Guajira was conducted at the recently discovered roosts, where the largest colony mainly consisted of pregnant females. On Tierra Bomba Island, the colony uses the fortresses only during the mating season (October–February) and leaves afterward (A. Otálora-Ardila, unpublished data). These reproductive behaviors may explain the large aggregations and high abundance observed of L. curasoae in northern Colombia. In contrast, no roosts were found for L. curasoae in the Chicamocha River Basin.
The high abundance of L. curasoae in the northern part of its range might also be linked with food availability. Stenocereus griseus and Cereus repandus, two columnar cacti species that are primary food resources for L. curasoae, have longer flowering and fruiting periods on the coast of Venezuela compared to the continental and intra-Andean arid areas of Venezuela and Colombia (Sosa and Soriano 1996; Ruiz et al. 2000; Nassar and Emaldi 2008). These differences in cacti reproductive patterns are probably mediated by variations in rainfall regimes between the coast and continental arid zones (Nassar and Emaldi 2008). Although specific data on reproductive phenology of cacti in the north of Colombia is lacking, similar differences in resource availability might exist between the northern regions and intra-Andean arid zones.
Most of the known roosts of L. curasoae are in northern Venezuela and the ABC islands, regions characterized by abundant karst formations (Hoekstra et al. 2010). Consequently, have more potential for cave development than continental regions in northern South America. Only in the ABC islands, the karst extension is nearly 13,000 km2 (Day 2010). This high concentration of caves likely explains the large populations of L. curasoae in this part of its range. High capture rates have been recorded in caves of ABC islands and the Paraguaná Peninsula in Venezuela, with population estimates of 14,350 bats in Bonaire and 26,517 in Aruba (Simal et al. 2015, 2021). Our data suggest a similar pattern in Colombia, where a cave in La Guajira Peninsula hosts a maternity colony of around 10,000 bats. Although the two roosts we found in Tierra Bomba are human buildings (Otálora-Ardila et al. 2022a, b), they host approximately 700 individuals. These three shelters are the only roosts known for L. curasoae in Colombia. Despite exploring roosts in the Chicamocha River Basin, including La Macaregua cave, which was previously reported as L. curasoae roost (Marinkelle and Cadena 1972), we did not find this species in any cave or artificial roost in this region.
Our data indicated that the current EOO is smaller than previously known (Nassar 2015). Upon revisiting specimens at mammal collections, we identified some specimens with incorrect localization data, confirming that the southern location of L. curasoae corresponds to the Chicamocha River Basin. Although our results indicate a reduction in EOO and AOO, the current EOO and AOO are still above the thresholds of 100 km2 or 10 km2, respectively, which would be necessary to classify L. curasoae as an Endangered species. Based solely on the current EOO and AOO values, this species would be classified as Near Threatened or Least Concern. However, the A2 criteria applied to L. curasoae in the past (inferred population reduction where the causes of reduction may not have ceased, be understood, or be reversible) remain relevant. Additionally, the threats faced by L. curasoae and knowledge about its biology and population parameters vary across the countries where it is distributed. The situation is more critical in Colombia because there is limited knowledge of roosts and populational parameters. Moreover, the identified threats are present throughout its known distribution in Colombia and are expected to increase in the near future. Consequently, considering all these factors and adopting a conservative approach, we recommend that L. curasoae be maintained as Vulnerable (VU A2c).
Our data showed that the environmentally suitable areas of the potential distribution of L. curasoae correspond to only 9.4 % of the area presented in the IUCN Red List (Nassar 2015). This discrepancy arises because the Extent of Occurrence assessed by the IUCN overestimates the actual distribution because it includes an erroneous record located 170 km to the southwest, corresponding to an individual captured in the Chicamocha River Basin. Also, the Extent of Occurrence represents the area encompassing all the known occurrences of a taxon, which includes unsuitable or unoccupied habitats (IUCN 2012). In contrast, the potential distribution refines the area occupied by a species by delimiting it to suitable areas.
Niche-based species distribution model and threats for L. curasoae. Bioclimatic variables such as temperature and precipitation are recognized as environmental factors influencing the potential distribution areas of tropical bats (da Silva et al. 2018; Debata et al. 2019; Garbino et al. 2023). Specifically, temperature seasonality (Bio 4) and precipitation seasonality (Bio 15) significantly contribute to the potential distribution of other tropical nectarivorous bats associated with arid environments (Burke et al. 2019). These climatic variables are positively correlated with primary productivity, water availability, and phenology in tropical dry forests (Stan and Sanchez-Azofeifa 2019). For instance, temperature promotes flowering production in seasonally dry forests (Pau et al. 2013), while precipitation regimes influence the flowering and fruiting patterns of columnar cacti, which are primary dietary sources for L. curasoae (Nassar et al. 2003; Nassar and Emaldi 2008). Furthermore, temperature and precipitation contribute to modulate the species and functional composition of chiropterophilous cacti used by L. curasoae in the inhabited dry ecosystems (Ruiz et al. 2002; Nassar et al. 2013). Consequently, these two bioclimatic factors are key drivers of the seasonal availability of food resources for this species.
Additionally, suitable areas for L. curasoae were associated with low mean diurnal range, suggesting that temperature is an extrinsic factor that might exert physiological constraints on this species. As noted by Ortega-García et al. (2017), L. curasoae has the narrowest thermal niche among Neotropical nectar-feeding bats, ranging from 16.6 to 32.6 °C. Thus, areas with temperature stability and values within these limits provide optimal thermal conditions for this species.
The spatial distribution of threats affecting L. curasoae suggests that, despite the relatively small anthropized area (8.4 %), there are marked differences in protected area coverage and the impacts of other threats among countries. Our data indicate that wind farms, mining, and tourism pose important threats in Colombia. For instance, mining has had more impact, with approximately 90 % of Colombia’s coal production occurring at La Guajira, and 90 % of gypsum and 30 % of limestone mining operations located in the Chicamocha River Basin (UPME 2022, 2023a). Wind farms represent another potentially critical threat, particularly in the ABC islands and northern Colombia, where 67 wind farm projects are planned along the Caribbean coast and from the La Guajira Peninsula to northern Cesar state (UPME 2023b). Although the long-distance flying routes of L. curasoae in Colombia are not well known, it is likely that some of the planned wind farms could overlap with the presumed migratory corridor that this species might use from La Guajira Peninsula to the east towards the Caribbean coast and south towards the intra-Andean dry areas (UPME 2023b).
Our data indicated that only 22.2 % of the suitable areas of L. curasoae are within protected areas. Extending or creating new protected areas is a high-priority conservation action for dry forests (Sánchez-Azofeifa et al. 2014; da Silva et al. 2018; Prieto-Torres et al. 2018). We suggest the creation of new protected areas in La Guajira Peninsula, the Caribbean coast near Cartagena and Tierra Bomba Island, the intra-Andean arid zones of Colombia and Venezuela, and Lara and Falcón states in Venezuela. These efforts are crucial for preserving local bat populations and protecting critical mating and maternity roosts, stopover foraging grounds, and migratory routes.
Although SDM models are valuable tools in conservation planning, they have limitations. For instance, the SDM assumes that species are at equilibrium with the environment, and biotic interactions are usually not considered due to the Eltonian Noise Hypothesis (Peterson et al. 2011). Our study did not include cave occurrences in the SDM due to the limited number of known L. curasoae roosts and the scarcity of cave inventories. Future research should extend the use of SDM to examine how food resource availability, migratory patterns, and climate change could impact L. curasoae populations.
Implications for conservation. Nectar-feeding bats like L. curasoae play a crucial role in plant pollination and seed dispersal, essential for maintaining dry forests and arid habitats (Arita and Wilson 1987). Therefore, preserving L. curasoae will contribute to maintaining biotic interactions, plant diversity, and the ecosystem services this species provides in the dry and xerophytic ecosystems of the ABC islands, northern Colombia, and Venezuela. Although representation of dry forests within Colombia’s protected areas increased by 68.4 % from 2010 to 2020, these ecosystems remain the least represented in the Colombian protected areas' system (Corzo et al. 2023). Connectivity between protected areas is also lower in the Caribbean ecoregion due to human transformation and because it has the smallest remnants of natural dry forests (Castillo et al. 2020).
Given the possible migratory behavior of L. curasoae, we recommend not only expanding existing natural areas or creating new ones but also establishing conservation corridors (Belote et al. 2016) and a network of connected private reserves across political boundaries (Kark et al. 2009). Conservation corridors should be prioritized along protected areas on the Caribbean coast of Colombia and Venezuela and in Falcón and Lara states in Venezuela. This approach is crucial to guarantee the seasonal movements and connectivity of L. curasoae populations throughout its distribution.
Conserving mating and maternity roosts is crucial since L. curasoae is strongly cave-dependent (Cole and Wilson 2006). Currently, seven important areas for bat conservation (AICOMs by its acronym in Spanish) and four important sites for bat conservation (SICOMs by its acronym in Spanish) have been recognized by the Latin American and Caribbean Network for Bat Conservation (RELCOM, https://www.relcomlatinoamerica.net/) as of high value to protect areas and caves or human buildings that serve as maternity and mating roosts of L. curasoae (Bárquez et al. 2022). Despite these efforts, our study indicates that roost vandalism remains a significant threat, menacing the species in several locations. For instance, as we completed the preparation of this article, we received a report from the coordinators of the Venezuelan Program for Bat Conservation (PCMV, the Venezuelan node of RELCOM) of a presumed case of massive poisoning of the bat colonies roosting in the Butare Tunnel at Falcón State (Venezuela), one of the identified roots used by thousands of L. curasoae bats (ID L385, Table ST1; A. García-Rawlins, Asociación Civil Topotepuy, pers. comm.). More than 200 adults and pups of L. curasoae and Mormoops megalophylla were found dead on the floor of the tunnel and hanging on the walls (date of sighting May 31st, 2024); however, it is unknown what substance was used. Roosts discovered in Colombia are particularly sensitive because one of them is the only known maternity roost for L. curasoae, and the other two are used by a colony only during mating season. Since its rediscovery in 2020, this colony has returned in 2022 and 2023 (A. Otálora-Ardila, unpublished data). Consequently, these two roosts are vital to maintaining the presumed long-distance movements of this species between the western part of its distribution and the north. Therefore, it is critical to establish effective management actions to protect bat colonies in those sites (see examples in Simal et al. 2021).
Our findings suggest that L. curasoae populations along the Caribbean coast of Colombia and Venezuela and in intra-Andean dry areas face more threats than those in the ABC islands. In the latter, ongoing bat protection initiatives have been implemented or are in the process of being established, having as targets the main bat roosts identified in the three islands (Simal et al. 2021). Identifying roosts in intra-Andean areas, determining their lapse of use, and assessing routes of migratory movements between coastal and intra-Andean zones is crucial. This is particularly important as planned wind farm developments in Colombia (UPME 2023b) could disrupt bat navigation (Jonasson et al. 2024), and interfere with these not yet identified flight routes. We also recommend creating intergovernmental action plans to protect this bat species at a regional level, addressing gaps in knowledge and guiding conservation efforts in a coordinated way to protect L. curasoae, with special focus on protection of maternal and mating roosts. Despite being included in general national-level strategies focused on migratory species (MAVDT et al. 2009), L. curasoae lacks specific conservation measures (Rojas-Díaz and Saavedra-Rodríguez 2014) and is often omitted from strategies aimed at protecting pollinator species (MADS et al. 2021).
Acknowledgments
We thank PANACHI (Parque Nacional del Chicamocha), Reserva ProAves Cucarachero del Chicamocha, Reserva El Palomar, and Fundación Batis for the logistical support. We thank the staff of Parques Nacionales Naturales de Colombia (PNN Tayrona and PNN Macuira) and Dirección de Patrimonio y Memoria (Ministerio de Cultura) and La Escuela Taller Cartagena de Indias, especially M. Orduña Monsalve and D. Brenon for the logistical support and help provided. Y. Girnu and M. Gutiérrez helped with fieldwork. AO-A is grateful for the grant from the National Geographic Society (NGS-61996C-19). APC-R (No. CVU 269863) thanks CONAHCyT for her postdoctoral research grant. We also thank to Fábio Z. Farneda for his valuable insight and the Grupo en Conservación y Manejo de Vida Silvestre, UNAL for supporting our research on bats in Colombia.
Literature cited
Aiello-Lammens, M. E., et al. 2015. spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38:541–545.
Albesiano, S., O. Rangel Ch., and A. Cadena. 2003. La vegetación del cañón del río Chicamocha (Santander, Colombia). Caldasia 25:73–99.
Altringham, J. D. 2011. Bats: from evolution to conservation, 2nd ed. Oxford University Press, New York, U.S.A.
Anla. 2014. Resolución 0255, 14 March, 2014. Bogotá D.C., Colombia.
Araújo, M. B., and New, M. 2007. Ensemble forecasting of species distributions. Trends in Ecology and Evolution 22:42–47.
Arita, H. T., and D. E. Wilson. 1987. Long-nosed bats and agaves: the tequila connection. Bats 5:3–5.
Barbosa, A. M. 2015. fuzzySim: applying fuzzy logic to binary similarity indices in ecology. Methods in Ecology and Evolution 6:853–858.
Bárquez, R. M., et al. (eds.). 2022. Áreas y sitios de importancia para la conservación de los murciélagos en Latinoamérica y el Caribe. RELCOM, Tucumán, Argentina.
Belote, R. T., et al. 2016. Identifying corridors among large protected areas in the United States. PLOS ONE 11:e0154223.
Burgin, C. J., et al. 2018. How many species of mammals are there? Journal of Mammalogy 99:1–14.
Burke, R. A., et al. 2019. Species distribution modelling supports “nectar corridor” hypothesis for migratory nectarivorous bats and conservation of tropical dry forest. Diversity and Distributions 25:1399–1415.
Cadena, A., J. et al. 1998. Dieta de los murciélagos frugívoros en la zona árida del río Chicamocha (Santander, Colombia). Boletín Sociedad Concepción Chile 69:47–53.
Cañón, S., and F. Trujillo. 2014. Mastofauna. Pp. 199–224, in Biodiversidad en Cerrejón (L. Baéz and F. Trujillo, eds.). Carbones de Cerrejón, Fundación Omacha, Fondo para la Acción Ambiental y la Niñez, Bogotá, Colombia.
Castillo, L. S. et al. 2020. Connectivity of protected areas: effect of human pressure and subnational contributions in the ecoregions of tropical Andean countries. Land 9:239.
Cazalis, V et al. 2024. Accelerating and standardising IUCN Red List assessments with sRedList. Biological Conservation 298:110761.
Cole, F. R., and D. E. Wilson. 2006a. Leptonycteris curasoae. Mammalian Species 796:1–3.
Corzo, G., et al. 2023. Efectividad de las áreas protegidas del bosque seco tropical. Biodiversidad, umbrales de transformación, estado y tendencias de la biodiversidad continental de Colombia (L. A. Moreno and G. Andrade, eds.). Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Bogotá, Colombia.
Day, M. 2010. Human interaction with Caribbean karst landscapes: past, present and future. Acta Carsologica 39:137-146.
Debata, S., R. M. Panda, and S. K. Palita. 2019. Chiropteran diversity and the key determinants of their distribution in Eastern Ghats, India. Biodiversity and Conservation 28:2385–2404.
Díaz, S. et al. 2019. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 366:eaax3100.
Escribano-Avila, G. et al. 2017. Biodiversity patterns and ecological processes in Neotropical dry forest: the need to connect research and management for long-term conservation. Neotropical Biodiversity 3:107–116.
Fajardo, L. et al. 2005. Tropical dry forests of Venezuela: characterization and current conservation status. Biotropica 37:531–546.
Feng, X., et al. 2024. Rethinking ecological niches and geographic distributions in face of pervasive human influence in the Anthropocene. Biological Reviews 99:1481–1503.
Frick, W. F., T. Kingston, and J. Flanders. 2020. A review of the major threats and challenges to global bat conservation. Annals of the New York Academy of Sciences 1469:5–25.
Garbino, G. S. T., F. Pessoa da Silva, and L. Gonçalves da Silva. 2023. Distribution, habitat suitability, and revised morphological diagnosis confirm that the fruit bat Platyrrhinus recifinus is an Atlantic Forest endemic. Studies on Neotropical Fauna and Environment 58:344–355.
García, R. E., and M. I. Silva. 2013. Las ABRAE versus las áreas protegidas en Venezuela. Copérnico 8:27–29.
Global Biodiversity Information Facility (GBIF). 2022. https://www.gbif.org/. Accessed on 12 February 2022.
Google Scholar. 2022. https://scholar.google.com/. Accessed on 22 March 2022.
Griffiths, T. A., and A. L. Gardner. 2007. Subfamily Glossophaginae. Pp. 224–243, in Mammals of South America: Marsupials, Xenarthrans, Shrews, and Bats (Gardner, A. L., ed.). The University of Chicago Press.
Hernández, J., et al. 1992. Unidades biogeográficas de Colombia. P. 204, in La diversidad biológica de Iberoamérica I (Halffter, G., ed.). Instituto de Ecología, A.C., Mexico.
Hoekstra, J. M. et al. 2010. The atlas of global conservation: changes, challenges, and opportunities to make a difference. 1st ed. University of California Press, California, U.S.A.
IDEAM. 2010. Promedios climatólogicos 1980-2010. http://www.ideam.gov.co/web/tiempo-y-clima/clima. Accessed on 10 July 2023.
International Union for Conservation of Nature and Natural Resources. 2012. IUCN Red List Categories and Criteria: Version 3.1. 2nd ed. Gland, Switzerland and Cambridge, U.K
International Union for Conservation of Nature and Natural Resources. 2020. IUCN Red List 2017-2020 Report.
International Union for Conservation of Nature and Natural Resources. 2023. The IUCN Red List of Threatened Species. http://www.iucnredlist.org. Accessed on 11 December 2023.
Jonasson, K. A., et al. 2024. A multisensory approach to understanding bat responses to wind energy developments. Mammal Review 54:229–242.
Karger, D. N., et al. 2021. Global daily 1 km land surface precipitation based on cloud cover-informed downscaling. Scientific Data 8:307.
Kark, S., et al. 2009. Between-country collaboration and consideration of costs increase conservation planning efficiency in the Mediterranean Basin. Proceedings of the National Academy of Sciences 106:15368–15373.
Karra, K., C. et al. 2021. Global land use/land cover with Sentinel 2 and deep learning. Pp. 4704–4707. in 2021 IEEE International Geoscience and Remote Sensing Symposium IGARSS.
Kass, J. M. et al. 2023. wallace 2: a shiny app for modeling species niches and distributions redesigned to facilitate expansion via module contributions. Ecography 2023:e06547.
Kass, J. M., et al. 2018. Wallace: a flexible platform for reproducible modeling of species niches and distributions built for community expansion. Methods in Ecology and Evolution 9:1151–1156.
Latorre, J. P., O. Jaramillo R., and L. Corredor G. 2014. Atlas del sistema nacional de áreas protegidas continentales en Colombia. Parques Nacionales de Colombia, Colombia.
MADS, et al. (eds.). 2021. Plan de acción de la iniciativa Colombiana de polinizadores. Ministerio de Ambiente y Desarrollo sostenible, Bogotá, D.C, Colombia.
MAVDT, et al. (eds.). 2009. Plan nacional de las especies migratorias: diagnóstico e identificación de acciones para la conservación y el manejo sostenible de las especies migratorias de la biodiversidad en Colombia. Ministerio de Ambiente, Vivienda y Desarrollo Territorial-WWF. Bogotá, D.C, Colombia.
Marinkelle, C. J., and A. Cadena. 1972. Notes on bats new to the fauna of Colombia. Mammalia 36:50–58.
Martino, A., A. Arends, and J. Aranguren. 1998. Reproductive pattern of Leptonycteris curasoae Miller (Chiroptera : Phyllostomidae) in northern Venezuela. Mammalia 62:69–76.
Martino, A. M. G., J. O. Aranguren, and A. Arends. 2002. Feeding habits of Leptonycteris curasoae in northern Venezuela. The Southwestern Naturalist 47:78–85.
Mining and Energy Planning Unit. 2023. Informe de registro de proyectos de generación de electricidad. UPME No. 0520, NO. 0638 de 2027 y No. 0143 de 2016. Ministerio de Minas y Energía de Colombia. Bogotá, D.C, Colombia
Ministry of Commerce, Industry, and Tourism. 2022. Estadísticas territoriales de turismo. Ministerio de Comercio, Industria y Turismo. Bogotá, D.C, Colombia
Molinari, J., et al. 2012. Singularidad biológica e importancia socioeconómica de los murciélagos cavernícolas de la Península de Paraguaná, Venezuela, con propuestas para su conservación. Revista de Ecología en Latino América 17:1–40.
Nassar, J. 2015. Leptonycteris curasoae. The IUCN Red List of threatened species 2015: e.T11699A22126917. http://dx.doi.org/10.2305/IUCN.UK.2015-4.RLTS.T11699A22126917.en. Accessed on 2 December 2023.
Nassar, J., and U. Emaldi. 2008. Fenología reproductiva y capacidad de regeneración de dos cardones, Stenocereus griseus (Haw.) Buxb. y Cereus repandus (L.) Mill. (Cactaceae). Acta Botánica de Venezuela 31:495–528.
Nassar, J. M., and F. Simal. 2019. Contribución de los AICOMs y SICOMs a la conservación de la quiropterofauna de la Isla de Bonaire. Boletín Red Latinoamericana Conservación de Murciélagos 10:4–8.
Nassar, J. M., et al.. 2003. Dependence on cacti and agaves in nectar-feeding bats from Venezuelan arid zones. Journal of Mammalogy 84:106–116.
Nassar, J.M., et al.. 2013. Las cactáceas como elementos de caracterización de ambientes áridos y semiáridos en Venezuela. Pp. 97-123, in Recorriendo el paisaje vegetal de Venezuela: un homenaje a Volkmar Vareski (Medina, E. , O. Huber, J. M. Nassar, and P. Navarro, eds.). Ediciones IVIC, Miranda, Venezuela.
Newton, L. R., J. M. Nassar, and T. H. Fleming. 2003. Genetic population structure and mobility of two nectar‐feeding bats from Venezuelan deserts: inferences from mitochondrial DNA. Molecular Ecology 12:3191–3198.
Olson, D. M. et al. 2001. Terrestrial ecoregions of the World: a new map of life on earth. A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 51:933–938.
Ortega-García, S. et al. 2017. The thermal niche of Neotropical nectar-feeding bats: its evolution and application to predict responses to global warming. Ecology and Evolution 7:6691–6701.
Otálora- Ardila, A., et al. . 2022a. Colombia S-CO-003: Fuerte San Fernando. Pp. 280. in Áreas y sitios de importancia para la conservación de los murciélagos en Latinoamérica y el Caribe (Barquez, R.. L. F. Aguirre, J. M. Nassar, S. F. Burneo, C. A. Mancina & M. M. Díaz, eds.). Yerba Buena, Tucumán, Argentina.
Otálora- Ardila, A., et. al. 2022b. Colombia S-CO-004: Batería Ángel San Rafael. Pp. 281, in Áreas y sitios de importancia para la conservación de los murciélagos en Latinoamérica y el Caribe (Barquez, R., L. F. Aguirre, J. M. Nassar, S. F. Burneo, C. A. Mancina, and M. M. Díaz, eds.). Yerba Buena, Tucumán, Argentina.
Pau, S. et al. 2013. Clouds and temperature drive dynamic changes in tropical flower production. Nature Climate Change 3:838–842.
Peterson, A. T., et al. 2011. Ecological niches and geographic distributions (MPB-49). Princeton University Press.
Petit, S. 1995. The pollinators of two species of columnar cacti on Curaçao, Netherlands Antilles. Biotropica 27:538–541.
Petit, S. 1997. The diet and reproductive schedules of Leptonycteris curasoae curasoae and Glossophaga longirostris elongata (Chiroptera: Glossophaginae) on Curaçao. Biotropica 29:214–223.
Petit, S., A. Rojer, and L. Pors. 2006. Surveying bats for conservation: the status of cave-dwelling bats on Curaçao from 1993 to 2003. Animal Conservation 9:207–217.
Pizano, C., and H. García (eds.). 2014. El bosque seco tropical en Colombia. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH), Bogotá, D.C, Colombia.
Portillo-Quintero, C. A., and G. A. Sánchez-Azofeifa. 2010. Extent and conservation of tropical dry forests in the Americas. Biological Conservation 143:144–155.
Prieto-Torres, D. A., J. Nori, and O. R. Rojas-Soto. 2018. Identifying priority conservation areas for birds associated to endangered Neotropical dry forests. Biological Conservation 228:205–214.
Protected Planet. 2023. https://www.protectedplanet.net/en. Accessed on 31 July 2023.
R Core Team. 2022. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria.
Razgour, O., J. Hanmer, and G. Jones. 2011. Using multi-scale modelling to predict habitat suitability for species of conservation concern: the grey long-eared bat as a case study. Biological Conservation 144:2922–2930.
Rodríguez, J. P., et al. 2007. The application of predictive modelling of species distribution to biodiversity conservation. Diversity and Distributions 13:243–251.
Rodríguez, J. P., F. Rojas-Suárez, and D. Giraldo Hernández (eds.). 2010. Libro rojo de los ecosistemas terrestres de Venezuela. Provita, Shell Venezuela, Lenovo (Venezuela). Caracas, Venezuela.
Rojas-Díaz, V., and C. A. Saavedra-Rodríguez. 2014. Murciélagos migratorios de Colombia. Pp. 151–238, in Guía de las especies migratorias de la biodiversidad en Colombia. Insectos, murciélagos, tortugas marinas, mamíferos marinos y dulceacuícolas (Amaya-Espinel, J. D., and L. A. Zapata, eds.). Ministerio de Ambiente y Desarrollo Sostenible / WWF-Colombia, Bogotá, D.C, Colombia.
Ruiz, A., et al. 2000. Estudio fenológico de cactáceas en el enclave seco de la Tatacoa, Colombia. Biotropica 32:397–407.
Ruiz, A., J. Cavelier, M. Santos, and P. J. Soriano. 2002. Cacti in the dry formations of Colombia. Pp. 324–341, in Columnar cacti and their mutualists – Evolution, Ecology, and Conservation (Fleming, T. H., and A. Valiente-Banuet, eds.) The University of Arizona Press. Tucson, Arizona, Arizona, U.S.A.
Sánchez, F., and A. Cadena. 1999. Migración de Leptonycteris curasoae (Chiroptera: Phyllostomidae) en las zonas áridas del norte de Colombia. Revista Academia Colombiana de Ciencias 23:683–686.
Sánchez-Azofeifa, A., et al (eds.). 2014. Tropical dry forests in the Americas: ecology, conservation and management. CRC Press, Boca Raton, Florida, U.SA.
da Silva, U. B. T., et al. 2018. Species richness, geographic distribution, pressures, and threats to bats in the Caatinga drylands of Brazil. Biological Conservation 221:312–322.
Sikes, R. S., W. L. Gannon, and Animal Care and Use Commitee of the American Society of Mammalogists. 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy 92:235–253.
Simal, F. et al. 2015. Island–island and island–mainland movements of the Curaçaoan long-nosed bat, Leptonycteris curasoae. Journal of Mammalogy 96:579–590.
Simal, F. et al. 2021. Bat Inventories at caves and mines on the Islands of Aruba, Bonaire and Curaçao, and proposed conservation actions. Acta Chiropterologica 23.
Sosa, M., and P. J. Soriano. 1996. Resource availability, diet and reproduction in Glossophaga longirostris (Mammalia: Chiroptera) in an arid zone of the Venezuelan Andes. Journal of Tropical Ecology 12:805–818.
Soriano, P. J., A. Ruiz, and J. M. Nassar. 2000. Notas sobre la distribución e importancia ecológica de los murciélagos Leptonycteris curasoae y Glossophaga longirostris en zonas áridas andinas. Ecotropicos 13: 91-95
Stan, K., and A. Sanchez-Azofeifa. 2019. Tropical dry forest diversity, climatic response, and resilience in a changing climate. Forests 10.
Thuiller, W., B. et al. 2009. BIOMOD – a platform for ensemble forecasting of species distributions. Ecography 32:369–373.
UNEP-WCMC. 2024. Protected area profile for Latin America & Caribbean from the world database on protected areas.
UPME. 2022. Boletín estadístico 2018-2022. Unidad de Planeación Minero Energética (UPME), Bogotá, Colombia.
UPME. 2023a. Consulta de Datos. https://www1.upme.gov.co/simco/Cifras-Sectoriales/Paginas/Informacion-estadistica-minera.aspx. Accessed on 12 December 2023.
UPME. 2023b. Dashboard informe registro de proyectos de generación. https://app.powerbi.com/view?r=eyJrIjoiMmMyZmM1MGMtNzExZC00NzJlLTk5ODAtNWUyMzYxMGMwMGYzIiwidCI6IjMzZWYwNmM5LTBiNjMtNDg3MC1hNTY1LWIzYzc5NWIxNmE1MyIsImMiOjR9. Accessed on 12 December 2023
VertNet. 2022. http://vertnet.org/. Accessed on 10 February 2022.
Villero, D., et al. 2017. Integrating species distribution modelling into decision-making to inform conservation actions. Biodiversity and Conservation 26:251–271.
Voigt, C. C., and T. Kingston. 2016. Bats in the Anthropocene. Pp. 1–9, in Bats in the Anthropocene: conservation of bats in a changing world (Voigt, C., and T. Kingston, eds.). Springer.
Web of Science. 2022. http://www.webofknowledge.com. Accessed on 20 March 2022.
Associated editor: Alina Gabriela Monroy-Gamboa
Submitted: July 28, 2024; Reviewed: August 23, 2024
Accepted: September 5, 2024; Published on line: September 20, 2024
Table 1. Mean, median, maximum, and minimum of bioclimatic variables considered in the potential distribution model of L. curasoae.
|
Annual mean temperature (ºC) Bio 1 |
Annual mean diurnal range (ºC) Bio 2 |
Temperature seasonality (CV, %) Bio 4 |
Annual precipitation (mm) Bio 12 |
Precipitation of driest month (mm) Bio 14 |
Precipitation seasonality (CV, %) Bio 15 |
Precipitation of coldest quarter (mm) Bio 19 |
|
|
Minimum |
8.35 |
0.9 |
15.5 |
276 |
1.1 |
25.4 |
6.2 |
|
Median |
26.35 |
7.2 |
72.8 |
919.7 |
12.2 |
60.9 |
84.3 |
|
Mean |
25.91 |
6.69 |
71.13 |
902.5 |
20.18 |
58.39 |
122 |
|
Maximum |
29.05 |
11.3 |
113.6 |
2356 |
124.1 |
110.6 |
574.1 |

Figure 1. Records of L. curasoae used for the Species Distribution Model (SDM). New localities and types of roosts are shown. The Extent of Occurrence (EOO) assessed in this study and from the IUCN Red List (Nassar 2015) for L. curasoae are shown.
Table 2 . Potential distribution area of L. curasoae (extension and percentage), protected areas overlapped (number and percentage) with the potential distribution of L. curasoae, and percentage of potential distribution area under protection by country.
|
Country |
Potential distribution area (km2) |
% of potential distribution area |
Number and (%) of protected areas |
% of potential distribution area inside protected areas |
|
Aruba |
172.21 |
0.22 |
1 (0.75) |
0.04 |
|
Bonaire |
273.44 |
0.35 |
3 (2.26) |
0.06 |
|
Curaçao |
419.53 |
0.53 |
7 (5.26) |
0.03 |
|
Colombia |
18,454.38 |
23.28 |
44 (33.08) |
1.94 |
|
Venezuela |
59,936.35 |
75.62 |
78 (58.65) |
20.17 |
|
Total potential distribution inside protected areas |
22.23 |
|||
|
Total potential distribution without protection |
77.77 |
|||
|
Total potential distribution |
79,255.91 |
|
|
|
.jpg)
Figure 2. Potential distribution, presence of protected areas (WDPA), and threats (mining, tourism, vandalism, wind farms) for L. curasoae.
Supplementary material
https://mastozoologiamexicana.com/therya/index.php/THERYA/article/view/6140/1447