THERYA, 2025, Vol. 16(1):107-124 DOI:10.12933/therya-25-6150 ISSN 2007-3364
The components of predation in Culpeo Foxes (Lycalopex culpaeus), and the value of long-term observations
Douglas A. Kelt1*, Peter L. Meserve2, M. Andrea Previtali3, W. Bryan Milstead4, Brian K. Lang, Hector Véas5, 6, Julio R. Gutiérrez6,
Alejandra J. Troncoso6, 7, and Madan K. Oli8
1 Department of Wildlife, Fish, & Conservation Biology, University of California, Davis, California 95616 USA. Email: dakelt@ucdavis.edu (DAK).
2 Department of Biological Sciences, University of Idaho, Moscow, Idaho USA. Email: pmeserve@uidaho.edu (PLM).
3 Departamento de Ciencias Naturales, Universidad Nacional del Litoral, Santa Fe, Argentina. Email: andrea.previtali@gmail.com (MAP).
4 U.S. Environmental Protection Agency, National Health and Environmental Effects Laboratory, Atlantic Ecology Division, Narragansett, RI 02882, USA. Email: willbmisled@gmail.com (WBM).
5 Bernalillo, New Mexico, USA (BKL).
6 Departamento de Biología, Universidad de La Serena, La Serena, Chile, and Centro de Estudios Avanzados en Zona Arida (CEAZA), La Serena, Chile (JRG). Email: hector.veas@gmail.com (HV), matroncoso@userena.cl (AJT).
7 Instituto de Ecología y Biodiversidad (IEB), Santiago Chile (JRG). Email: matroncoso@userena.cl (AJT)
8 Department of Wildlife Ecology and Conservation, University of Florida, Gainesville, Florida 32611 USA. Email: olim@ufl.edu (MKO).
*Corresponding author: https://orcid.org/0000-0001-8254-9117.
Understanding predator-prey dynamics requires insight on predator responses to variation in prey abundance. Whereas most predators respond numerically to changes in food availability, functional responses are less clear. Characterizing both is essential to understanding how predator-prey dynamics will change with spatiotemporal variation in resources or habitat conditions. The Culpeo (Lycalopex culpaeus) is a wide-ranging South American canid broadly characterized as a generalist forager exhibiting numerical responses, but limited functional responses, to variation in prey availability. We employ a 23-year perspective on the diet of Culpeos, using monthly demographic monitoring of small mammal prey species and concurrent collection of Culpeo scat in a large protected area in Chile. As elsewhere, Culpeos emphasize small mammals in their diet, but they consistently consume some species (notably Abrocoma bennettii) disproportionate to their apparent availability. Culpeos here display limited numerical responses to variation in small mammal abundance, although this weakens during extended periods of low small mammal availability when foxes presumably switch to other food items. Culpeos exhibit an asymptotic (Type II) functional response to variation in abundance of small mammal prey. While Type II functional responses are generally considered to characterize specialist predators, these patterns match expectations for a generalist forager that strongly favors certain prey species, and underscore the importance of long-term data for elucidating fundamental ecological patterns. Further work is needed to dissect this functional response among key prey species, and to determine if and how this may fluctuate with climatic conditions, which vary extensively here due to El Niño Southern Oscillations. Long-term datasets provide unique opportunities to understand and characterize such patterns in natural communities.
Entender la dinámica depredador-presa requiere comprender cómo los depredadores responden a las variaciones en la abundancia de presas. Mientras la mayoría de los depredadores responderán numéricamente a los cambios en la disponibilidad de alimento, las respuestas funcionales son menos claros. Caracterizando ambas respuestas es esencial para comprender cómo cambiara la dinámica entre depredador y presa a la variación espaciotemporal en recursos o en las condiciones del hábitat. El Culpeo (Lycalopex culpaeus) es un cánido sudamericano ampliamente distribuido y generalmente caracterizado como un depredador generalista exhibiendo respuestas numéricas, pero limitada (o no) respuestas funcionales, a la variación en la disponibilidad de presas. Nosotros empleamos una perspectiva de 23 años en la dieta de Culpeo, usando un programa de monitoreo demográfico mensual de micromamíferos y la colección simultánea de fecas de culpeo en un área protegida en Chile. Como en otras partes, los culpeos seleccionan micromamíferos en su dieta, pero consumen algunas especies (especialmente Abrocoma bennettii) en forma desproporcionado en relación con su disponibilidad aparente. Culpeos acá muestran una respuesta numérica limitada a la variación en abundancia de micromamíferos, aunque esto es más débil durante largos períodos de disponibilidad baja de micromamíferos cuando los zorros probablemente cambian a otros alimentos. Los culpeos exhiben una respuesta funcional asintótica (tipo II) a la variación en la abundancia de presas de micromamíferos. Tanto como respuestas funcionales tipo II son generalmente considerado a caracterizar depredadores especialistas, estos patrones coinciden con la expectativa de un forrajeo generalista que fuertemente favorece ciertas especies de presa y destacan la importancia de los datos a largo plazo para elucidar patrones ecológicos fundamentales. Se necesitan más trabajos para disectar esta respuesta funcional entre especie de presas clave y para determinar si y cómo esto puede fluctuar con las condiciones climáticas, que pueden variar ampliamente en asociación con la Oscilación del Sur El Niño. Conjuntos de datos a largo plazo proporcionan oportunidades únicas para entender y caracterizar estos patrones fundamentales en las comunidades naturales.
Keywords: Diet; foraging ecology; functional response; generalist predator; long-term data, numerical response.
© 2025 Asociación Mexicana de Mastozoología, www.mastozoologiamexicana.org
Introduction
In simplest terms, predator foraging theory predicts that predators should respond to variation in prey availability numerically and/or functionally (e. g., changes in numbers of predators versus changes in per-capita prey consumption—Holling 1959; Jaksic et al. 1993). Numerical responses may occur over the short term (immigration and emigration) or longer term (local recruitment or mortality), whereas functional responses are more immediate. Because generalist predators are likely to switch prey and to develop search images for favored prey as they increase in abundance, their functional responses should be sigmoidal in shape (e. g., Type III), with a lagged response as prey numbers increase, followed by increased per capita consumption to some point of satiation. Specialist predators, on the other hand, switch prey less frequently (or among fewer target prey) and are more likely to exhibit Type II functional responses (e. g., with no lag period). However, these functional responses comprise points on a continuum, and we should anticipate variation in the extent to which predators match these patterns (Denny 2014). In general, smaller predators tend to be more generalist and opportunistic in their diet than larger predators, which tend to forage on more prey species but more selectively (Gittleman 1985).
Small canids tend to be opportunistic foragers, and may exhibit either specialist or generalist prey selection. Arctic Foxes (Vulpes lagopus) in Siberia act as specialists on Norwegian Lemmings (Lemmus lemmus), but as generalists on Collared Lemmings (Dicrostonyx torquatus; Angerbjorn et al. 1999); in Fennoscandia this canid is a lemming specialist, but opportunistically consumes birds, Reindeer (domestic Rangifer tarandus, presumably carcasses), other small mammals, and hares (Elmhagen et al. 2000). Red Foxes (V. vulpes) are “the archetypical generalist predator” (O'Mahony et al. 1999:575), and consume a broad array of food items across an immense geographical range, as do both Golden (Canis aureus; Aleksandra and Dusko 2015) and Black-backed jackals (Lupulella mesomelas; Humphries et al. 2016). Reflecting this trophic flexibility, the diet of some smaller and mid-sized canids varies regionally (e. g., Etheredge et al. 2015).
Two wild canids range over much of the Southern Cone of South America. These are the larger Culpeo Fox (Lycalopex culpaeus, 4 to 14 kg; Culpeo hereafter) which ranges along the Andes from Colombia to Tierra del Fuego (excluding wet temperate rainforest; Novaro 1997b), and the smaller and competitively subordinate Chilla Fox (L. grisea, 2 to 5 kg; Chilla hereafter), which ranges from southern Perú to southern Patagonia (introduced to Tierra del Fuego—Jaksic and Yáñez 1983; Lucherini 2016). Additionally, the Pampas Fox (L. gymnocerca) ranges from eastern Bolivia and western Paraguay south through the Monte, Espinal, and Pampas ecoregions of Argentina (Lucherini and Luengos Vidal 2008), and Darwin’s Fox (L. fulvipes) has a very restricted range in south-central Chile (Iriarte and Jaksic 2012). The most widely distributed of these, the Culpeo and Chilla, have been studied extensively (reviewed by Jaksic 1998) and both tend to have diverse diets (as does the Pampas Fox; Lucherini and Luengos Vidal 2008). In the coastal fringe of the Atacama Desert, Culpeos consume primarily arthropods, complemented by reptiles, birds, and small mammals (Guzmán-Sandoval et al. 2007); Chillas in this area target small mammals but also consume seabirds and crustaceans (Marquet et al. 1993). In the southern Andes the Culpeo consumes small mammals (Marquet et al. 1993), but this may be complemented by camelids (presumably carrion), European Hares (Lepus europaeus), and birds, leading Walker et al. (2007) to conclude that Culpeos were more generalist than other mid-sized carnivores in the region (e. g., Andean Mountain Cat [Leopardus jacobita], and Colocolo [L. colocola]). Where both canid species co-occur in Patagonia, Chillas emphasize arthropods and small mammals in their diet while Culpeos focus on invasive European Hare (Zapata et al. 2005; Gantchoff and Belant 2016); Palacios et al. (2012) further emphasized the role of invasive species in the diet of Culpeo in this region, arguing that native prey items are ecologically (hence, functionally) extirpated in this region. In steppe habitat of central Argentina, Culpeos appear to retain a preference for native small mammals (by biomass; Pia et al. 2003), whereas in mediterranean Chile they appear to have adopted European Rabbits (Oryctolagus cuniculus) as their primary prey (Rubio et al. 2013), and in forested regions of southern Chile both canids (as well as Darwin’s Fox) shifted their apparent prey preferences in exotic plantations (Monterrey Pine, Pinus radiata) relative to native forest, selecting arboreal rodents (Oligoryzomys longicaudatus, Irenomys tarsalis) in the former even though these were more abundant in the latter (Moreira-Arce et al. 2015).
This brief overview underscores the adaptability of Culpeo in the face of changing biotic conditions. Efforts to characterize either numerical or functional responses, however, have been limited to two sites in northern Chile. At an interior site in the northern mediterranean region (Las Chinchillas National Reserve), Jaksic et al. (1992) and Martínez et al. (1993) tracked fox numbers and diet over 45 months and one cycle in prey abundance (mostly native small mammals; invasive lagomorphs had not yet become abundant at this site; F. Jaksic pers. comm. to DAK, October 2016). Jaksic et al. (1992:95) reported that Culpeos “consumed highly variable amounts of fruit regardless of the availability of mammalian prey,” and while Martínez et al. (1993) did not distinguish the scat of Culpeo and Chilla, the combined dietary information showed no indication of a functional response; rather, foxes exhibited strong preference for Common Degus (Octodon degus; Degu hereafter) and Darwin’s Leaf-eared Mice (Phyllotis darwini). At a coastal site ca. 90 km from Las Chinchillas (Bosque Fray Jorge National Park; Fray Jorge hereafter), Jaksic et al. (1993, 1997) reported somewhat similar dynamics; Culpeos (the only fox present at that site) were selective predators, favoring Bennett’s Chinchilla Rat (Abrocoma bennettii) and Degus, but avoiding P. darwini, although they increased consumption of fruits when small mammal numbers were low (Castro et al. 1994). Finally, whereas Jaksic et al. (1993) reported no numerical responses across variation in prey abundances, Jaksic et al. (1997) suggested a modest numerical response, although this was confounded by the fact that these authors combined multiple predator species (Culpeo and raptors) in their assessment of numerical responses. Neither study at Fray Jorge (Jaksic et al. 1993, 1997) suggested functional responses by Culpeos, which continued to emphasize small mammals throughout the study period, albeit complemented occasionally by arthropods and fruit/seed.
One potential complication in a region known for extensive temporal variation in abundance of prey species (Iriarte et al. 1989a; Jiménez et al. 1992; Meserve et al. 2016) is that all studies on the diet of the Culpeo have been limited in the temporal extent of observation. We have been studying the ecology of small mammals at Fray Jorge since 1989, including monthly assessments of population sizes at a series of replicate study plots (reviewed in Meserve et al. 2003, 2016; Gutiérrez et al. 2010) and complemented with monthly collection of Culpeo scat along standardized routes. This is the same site reported on by Jaksic et al. (1993, 1997), but here we report on a much longer (23-year) dataset spanning extensive temporal variation in small mammal numbers, and simultaneous variation in Culpeo diets.
Two events at this site prompted this updated analysis. First, climatic patterns appear to have shifted in about 2000/2002. Prior to this, the site experienced periodic El Niño Southern Oscillation wet periods every 3 to 5 years, with strong numerical responses by small mammals (Meserve et al. 1993, 1996). After about 2002, annual rainfall declined and became less variable (Meserve et al. 2011, 2016; Armas et al. 2016a); subsequent to this climatic change, we documented a major shift in the composition of the small mammal fauna, the most notable change being a tremendous proportional increase in Degu abundance. Since 2001 this species has comprised at least 50 % of the biomass of small mammals at our site, and over 65 % since 2010 (Meserve et al. 2016). If Degus are a favored prey of Culpeo (Jaksic et al. 1993, 1997), then we would predict that this predator would respond to this change in prey availability.
The second major change at the site is that invasive lagomorph populations have increased greatly, starting in late 2002 or early 2003. This appears to be a response to two unrelated events. The first of these was that 2000 to 2002 comprised a series of three years of elevated rainfall at our site, leading to abundant food for herbivorous or folivorous consumers. Additionally, Culpeos at Fray Jorge experienced a distemper outbreak in 2002/03 (Moreira and Stutzin 2005; Acosta Jamett 2010) which led to a substantial decline in fox numbers and presumably in predation pressure on lagomorphs. In our field notes from that time period we document encountering numerous fox carcasses, and for the first time noting both European Hares and European Rabbits commonly. Hares and rabbits have remained abundant throughout the park since this time (DAK, pers. obs.).
Given the existing literature on the population ecology of Culpeos, we predicted that they would respond numerically but not functionally to changes in prey abundance. We predicted that Culpeos would favor some species as prey (e. g., larger small mammals but also lagomorphs once the latter became abundant at our site), but that they would show no sign of adjusting their foraging to changes in prey availability.
Materials and methods
Study site. Since 1989 we have maintained a long-term study on the ecology of arid lands in Fray Jorge, at the northern fringe of mediterranean Chile (reviewed in Squeo et al. 2004; Armas et al. 2016b). Initially established to assess the relative importance of biotic influences (competition and predation) on small mammals (Meserve et al. 1993) and top-down impacts on plants (Gutiérrez et al. 1997), we have refocused effort to target the relative importance of biotic versus abiotic drivers after episodic rainfall, generally associated with the El Niño Southern Oscillation (ENSO) phenomenon. Such events effectively “reset the clock” of predator-prey dynamics, with rainfall promoting primary productivity and providing abundant food for small mammals, effectively releasing them from any top-down control by predators (Meserve et al. 2003, 2016). After multiple wet/dry cycles we have documented predictable responses by most biotic elements, and research emphasis has shifted to monitoring diverse biotic and abiotic parameters in the face of the apparent “regime change” (sensu Scheffer 2009) in 2000/02.
The main part of Fray Jorge comprises about 9,000 ha on the coast of north-central Chile, located approximately 400 km North of Santiago and 150 km South of the southern border of the Atacama Desert. The region is semi-arid but receives a strong oceanic influence. Coastal hills (the Altos de Talinay, ca. 640 m elevation) intercept fog and support remnant patches of forest with numerous elements of Valdivian temperate rainforest, characteristic of southern Chile (Villagrán et al. 2004; Squeo et al. 2016). Our study is in the Quebrada de las Vacas, a north-south oriented valley located just interior to the Altos de Talinay, and dominated by spiny drought-deciduous and evergreen shrubs with a seasonal ephemeral plant understory (Gutiérrez et al. 1993; Gutiérrez et al. 2004; Squeo et al. 2016). The climate is semi-arid Mediterranean with warm summers and cool winters. Mean annual precipitation is just under 127 mm, most of which falls in winter (May to October).
The small mammal community of Chile is well known (Osgood 1943; Jaksic 1998; Muñoz-Pedreros and Yáñez 2009), and the fauna of Fray Jorge has been well studied since the 1970s (Schamberger and Fulk 1974; Fulk 1975; Meserve 1981a, b: Meserve et al. 1993, 1995, 1996, 2001, 2003, 2016; Yunger et al. 2002; Kelt et al. 2004a, b, c). The community is diverse and includes three herbivorous caviomorph rodents (Octodon degus [ca. 120 to 180 g], O. lunatus [ca. 160 to 200 g], and Abrocoma bennettii [150 to 250 g]) and several smaller (20 to 80 g) sigmodontine rodents. The most abundant sigmodontines are the omnivorous Abrothrix olivacea and omnivorous / herbivorous Phyllotis darwini, but lesser numbers of insectivorous A. longipilis and granivorous Oligoryzomys longicaudatus are recorded as well (dietary characterization from Meserve 1981a). Finally, a carnivorous / frugivorous mouse opossum (Thylamys elegans, 25 to 35 g) occurs here and is regularly captured. We classify species as “core”, “quasi-core”, or “opportunistic”, based on their apparent dependence on thorn scrub habitat, where our sampling is based. Octodon degus, P. darwini, and A. olivacea are captured every month and are considered to be core species. Thylamys is captured in most months but with less consistency, and we frequently treat this as a quasi-core species. All of these species may also be found in other key habitats at Fray Jorge, including fog forest that occurs on the adjacent Altos de Talinay and in moister aguadas habitat, where the water table is sufficiently high to support more mesic vegetation. Supplemental trapping in these habitats (Milstead et al. 2007) suggests that A. longipilis is primarily a species of the fog forest, but it enters thorn scrub habitat when conditions facilitate population expansion, such as during rainy periods. Oligoryzomys also appears to favor moister habitats (both fog forest and aguadas), as does O. lunatus. Because these species may disappear from our thorn-scrub trapping grids for months at a time, we consider these to be opportunistic residents. Further information on these species and the broader structure of our research program may be found in Armas et al. (2016b).
Small mammal abundances. Small mammals have been surveyed monthly on 16 to 20 replicate plots allocated to 4 to 6 experimental treatments that include selective exclusion of predators or subsets of the small mammal assemblage (Kelt et al. 2013; Meserve et al. 2016). For the purposes of this study we only use data from four control plots that have not been subjected to any biological exclusion through the duration of the study. All plots are 75 x 75 m in area, and control plots are encircled with a low fence (ca. 0.6 m) using “chicken-wire” (2.5 cm hexagonal mesh) to which we facilitate access by all small mammals by cutting 5 cm dia. holes at 5-m intervals (Meserve et al. 1993). Each plot includes a 5 x 5 trapping grid, with 15-m spacing between stations. Monthly surveys comprise four consecutive nights and days using 50 large Sherman-type live traps (10.5 x 11.3 x 30.5 cm; two traps per station). All captured animals are identified to species and uniquely marked with numbered eartags or leg bands. All field efforts meet criteria established by the American Society of Mammalogists (Sikes and the Animal Care and Use Committee of the American Society of Mammalogists 2016) and have been approved by Institutional Animal Care and Use Committees at our respective institutions.
We used the superpopulation model (Schwarz and Arnason 1996; Williams et al. 2002) to estimate population size for small mammal species occurring on our control grids (n = 4) from December 1990 through August 2013. To match seasonal dynamics at Fray Jorge we calculated mean values over 3-month periods (Summer, Dec to Feb; Fall, Mar to May; Winter, Jun to Aug; Spring, Sep to Nov.), although in some presentations we employ 6-month periods (reproductive, Sep to Feb; non-reproductive, Mar to Aug; hence, reproductive season “R1990” would span September 1990 through February 1991). Demographic modeling was conducted in Program MARK (White and Burnham 1999) using the RMark package (Laake 2013) for the R computing environment (R Core Team 2024), as detailed in Kelt et al. (in prep).
Numerical responses by Culpeo. To assess Culpeo activity at our site we established three olfactory lines (21 scent stations each, approximately 100-m intervals) among the small mammal trapping plots (Previtali et al. 2009). Each station consisted of a ca. 1 m dia. plot of sifted and smoothed sand that was cleared of vegetation. We surveyed for fox activity ≥2 days per month by placing a cotton-wrapped stick soaked in predator lure (Bobcat #1 lure; Cronk’s Outdoor Supplies, Wiscasset, Maine, USA) in the center of each station. Following Previtali et al. (2009) we estimated fox activity as the number of scent stations visited (based on scratch marks, tracks, removed lure, etc.) during the first two days of olfactory surveys. Data were tabulated in 3-month windows for comparison with small mammal population estimates.
Culpeo diet. Culpeo scat have been collected monthly at our site since March 1989. Scat are collected in the Quebrada de las Vacas where all of our small mammal censuses were conducted. As noted by Jaksic et al. (1992), deterioration over a single month is trivial in this arid region, and sampling on established routes ensured that sampling intensity remained approximately equal in all months. After drying, scat were physically separated and all items identified by trained technicians, using a dissecting microscope as needed. For animal prey other than lagomorphs, we determined the minimum number of individuals of each taxon on the basis of identifiable structures (e. g., mandibles, crania, antennae, wings, etc.). Most lagomorph remains consisted of bone fragments and occasional teeth, effectively precluding estimation of the number of individuals in a given scat.
We tested for seasonal differences in diet (reproductive vs. non-reproductive) for basic dietary groups (mammals, birds, etc.) using t-tests. The only groups suggesting seasonal differences were ectothermic (reptiles) or partially migratory (birds); because these are minor elements of the diet of Culpeos (representing, on average, 6 and 5 %, respectively, of prey remains in seasonal surveys) and we lack estimates of actual densities for comparison against frequency of consumption, we omit these groups from further consideration. Because small mammals dominate the diet (see Results) and because we have contemporaneous estimates of population density, we restrict our attention to these prey items in most of the following analyses.
We calculated basic descriptive parameters for each seasonal diet sample. These included proportion of items comprising key species, number of scat sampled, and three metrics of diet composition – diet breadth, diet diversity, and diet evenness. Following Jaksic et al. (1992, 1993) diet breadth was calculated as B = 1 ⁄ ∑pi2 where pi is the proportional representation of prey item i in the diet during a given time period. With n diet categories, B ranges between 1 and n, and provides an index of resource use that is independent of relative availability (Feinsinger et al. 1981). We calculated diversity with the Shannon diversity index (H'= ∑pi [ln(pi)] which ranges from 0 to ln(S) where S = the number of species present (Magurran 1988). Finally, evenness was calculated as E = H’/ln(S). E ranges from 0 to 1, with higher values reflecting increased numerical similarity across categories (Magurran 1988). We applied these metrics at two scales of analysis, first assessing general patterns of consumption across broad trophic categories (e. g., mammal, bird, etc.) and subsequently assessing consumption by different species of small mammals (both with and without invasive lagomorphs, see below).
We characterize prey species as “selected” when their frequency in scat samples (see Supplementary material Table S2) exceeded their relative abundance based on live trapping, and as “avoided” or “negatively selected” when the frequency in scat was less than their relative abundance based on live trapping (see Supplementary material Table S3). We conservatively tested for significant deviation from random variation with a binomial test of the number of periods in which ratios of selection or avoidance were ≥1.5.
Non-native lagomorphs. European Hares and European Rabbits have been present in mediterranean Chile for decades (Camus et al. 2008; Jaksic and Castro 2014), but neither was markedly abundant at Fray Jorge until about 2003, following three sequential years of above-normal rainfall and a distemper outbreak that greatly reduced the Culpeo population (Moreira and Stutzin 2005; Acosta Jamett 2010). Once Culpeo numbers declined, both species of lagomorphs became notably abundant, but this was particularly true for Oryctolagus. Fox numbers rebounded in subsequent years (DAK, pers. obs.), but because of the increase in lagomorph remains in fox scat in several years (e. g., 2003 to '07, 2011) we calculate diet breadth and diversity both with and without lagomorphs.
Lagomorphs typically are surveyed with live-trapping or distance sampling methods (e. g., Palomares 2001). Unfortunately, European Hares and European Rabbits are not readily live-trapped, and extensive efforts with distance methods have proven fruitless at this site, where high shrub cover (>50 %) limits visibility, and even nocturnal spotlighting efforts yield very few individuals. Therefore, in 2008 we established a series of 54 pellet-count stations (cf. Murray et al. 2002) to obtain standard indices of lagomorph numbers. Each station consists of a 1-m circular area cleared of vegetation and existing pellets. We placed a stake at the center of each station and at ca. 6-mo intervals (generally August and February/March) we counted and removed all pellets present. This interval conformed to the timing of site visits by the principal investigators. Although pellets of adult Lepus are readily distinguished from those of adult Oryctolagus, variation in pellet size reflecting the size and age of the animal precluded separate metrics for each taxon; instead, we present a single metric of lagomorph activity, although field observations make it clear that Oryctolagus is much more abundant than Lepus.
In 2011 we established a second effort to survey lagomorphs, consisting of 21 stations at ca. 500-m intervals along the only dirt road in our study site. At each station we cleared vegetation and sifted fine soil over two ca. 1.5-m dia. circular plots located 3 to 5 m apart. At the center of each plot we placed a single Petri dish filled with approximately 2 to 5 mm plaster of Paris. Upon placement, we added about 4 to 5 drops of scent, consisting of either mint or carrot extract (one of each at every station) dissolved in propylene glycol to inhibit evaporation. Stations were smoothed with a fine brush, and dishes placed in the evening, and checked early in the morning for tracks or pellets. We surveyed stations for four consecutive nights, replacing scent each evening. With 8 ½ years of data for both survey methods, total pellet counts (excluding the extreme high value from each season) and olfactory plot visits exhibit very high correlation (F1,15 = 12.15, P = 0.0033), although there remains noise in these data (adjusted R2 = 0.41). While neither metric can be tied to actual lagomorph numbers, the agreement between these independent assays suggests that they provide a useful index of lagomorph abundance.
Functional and numerical response. We follow Jaksic et al. (1996, 1997) in assessing numerical responses by Culpeos to variation in small mammal abundance by regressing the abundance of foxes (as inferred from olfactory lines; Previtali et al. 2009) against small mammal population density (on control plots). Theory predicts a counter-clockwise trajectory of data in such a bivariate plot; as small mammal numbers (x axis) increase, at some point consumption by foxes (y axis) should increase, and this pattern should reverse as small mammal numbers decline. Such patterns frequently are challenging to quantify, however, given large interannual variation in prey abundances.
We assessed functional responses to variation in small mammal prey items in two ways. First, we characterized patterns of prey consumption by comparing consumption of different small mammal species and associated dietary metrics (breadth, diversity, evenness) as functions both of sample sizes (number of scat analyzed) and of prey population size. Second, we plotted our best estimate of per capita consumption (e. g., the mean minimum number of prey items per scat) against population size across 45 6-month sampling periods. We characterized Type I, II, and III functional responses with linear, quadratic, and sigmoidal non-linear regression, respectively, which we compared against a null model corresponding to a constant value of the mean number of prey items consumed across all prey densities. We compared regression models with Akaike’s Information Criterion corrected for small sample size (AICc), and considered models to be competitive if they had Akaike differences (ΔAICc) ≤ 2.0 and relatively high Akaike weights (wi).
Results
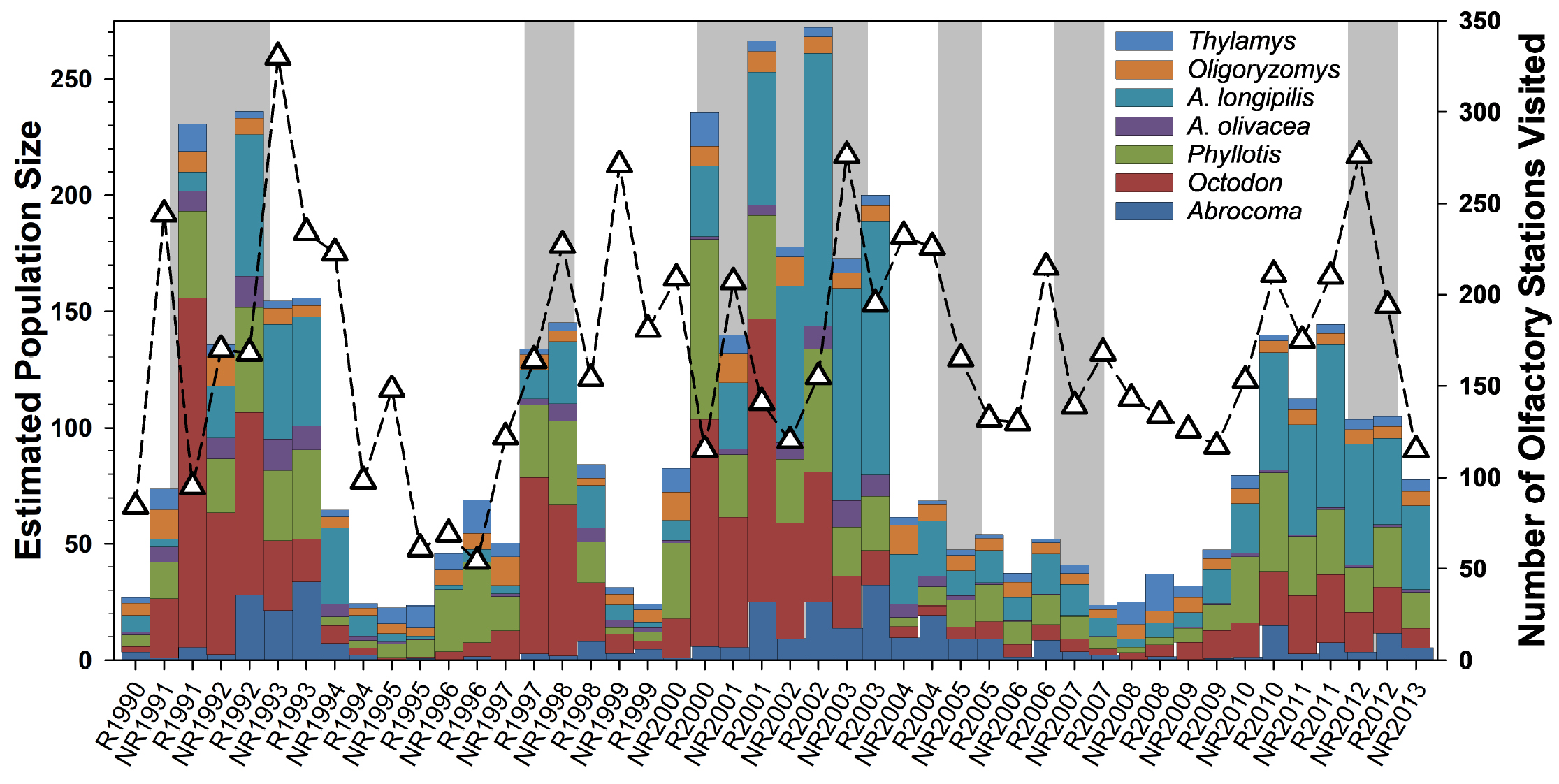
Temporal patterns in small mammal numbers and Culpeo activity. Small mammal populations have fluctuated greatly over time and generally are strongly influenced by rainfall (Meserve et al. 2016; Figure 1). The greatest numerical changes were documented for Abrothrix olivacea, Octodon degus, and Phyllotis darwini, with lesser changes by most other species. Culpeo activity at our site, as measured by the number of olfactory stations visited, varied over the duration of our study, and generally increased during and after rainy periods (Figure 1), but in general these data suggest that fox activity fluctuated substantially around a long-term mean (ca. 170 station visits monthly) through the study period.
Culpeo dietary selectivity. We analyzed 4,769 scats collected over 23 years (Supplementary material Figure S1, Table S1). These contained ≥8,297 individual animal prey items, 5,128 (61.8 %) of which were mammals, 468 (5.6 %) birds, 373 (4.5 %) reptiles, and 2,328 (28.1 %) invertebrates. It is clear, however, that mammalian prey were both the dominant and most consistent food consumed; arthropods comprised significant components of the diet only in about 12 of 46 6-month periods (Figure 2a), and their biomass contributions were substantially less than that of small mammals. We also tallied 261,703 seeds (Figure 2b), supporting earlier observations that Culpeo consume considerable fruit (Jaksic et al. 1992; Castro et al. 1994), although these appear to be somewhat sporadic in occurrence in scats (Figure 2b).
Because we have data on both use (consumption) and availability (control grids) of small mammals we are able to assess selectivity of these prey by foxes. The proportional representation of seven species of small mammals was relatively invariant across both the number of scat collected (one metric of fox activity; Figure 2c) and total small mammal abundance (an index of prey availability; Figure 2e), although these data indicate clearly that Abrocoma and Octodon comprise the majority of mammalian food items consumed by Culpeos (Figure 2c, 2e). Three dietary metrics (breadth, diversity, evenness) exhibited trivial variation with respect to the number of scat sampled (Figure 2d), and while all three varied significantly as a function of small mammal population size (Figure 2f), they explained less than one-third of the variance in the available data. Perhaps most notably, Culpeo diet breadth regressed positively on small mammal population size; Culpeos tended to eat fewer species when prey populations were low, and more species when these populations were high. The relative contribution of lagomorphs is more difficult to assess, because we lack estimates of their abundance for most of the study period, and the number of items in scat do not necessarily represent separate individuals. However, Figure 2a suggests that lagomorphs have become more common in Culpeo diets since about 2003.
Seasonal analysis of Culpeo diet provides greater resolution of prey selectivity (Figure 3). Most species of small mammals were consumed in greater proportion to their availability during some seasons and in lesser proportions in others. The only species consistently over-represented was the large herbivorous Abrocoma; this species was positively selected in 44 of 46 6-month seasons (Figure 3a, b). In contrast, A. olivacea, Oligoryzomys, Phyllotis, and Thylamys all were better represented in our monthly surveys than they were in Culpeo scat, therefore being selected against in most seasons, and selected for in just zero or one season each. In contrast, both O. degus and A. longipilis were selected for and against at roughly similar rates (11 and 13 seasons for the former, seven and five for the latter).
For those species that were positively selected, selectivity was greatest when those species were low in abundance (Figure 3, green symbols in right panels). There was some indication of similar patterns for avoidance (e. g., Octodon and Phyllotis, for example; red symbols) but this was less consistent than the pattern for positive selection.
Numerical responses. Using all data points (n = 101 3-month periods; Figure 4a) fox activity is only modestly associated with small mammal abundances (linear regression, P = 0.10) and the relationship explains little variation (R2 = 0.026) and has a very low slope (β = 0.021). However, of interest here is whether these data tend to comprise counter-clockwise cycles, with predators responding positively to higher small mammal populations, and negatively to lower populations. When these data are partitioned into sequential multi-year cycles the results are suggestive, although temporally variable as one might expect given interannual heterogeneity at this site (e. g., rainy versus dry years). However, in most cycles the data generally follow the expected counter-clockwise trajectory (Figures 4b-g). Years when this pattern is less clear (e. g., 2004 to 09, 2009 to 13) are relatively dry years in which small mammal abundances remained relatively low; under such conditions one would not expect substantial demographic response by Culpeos, and this was observed.
Functional responses. Whereas proportional consumption of broad trophic categories as well as different small mammal species in scat samples is effectively invariant across both Culpeo and small mammal abundance (Figure 2), per capita consumption of small mammals clearly increases with availability (Figure 5). This observation rejects our hypothesis of no functional response, leaving open the question of the nature of the functional response (e. g., Type I, II, or III). Perhaps surprisingly, the available data are not explained better by any of these models; AICc values are not greatly different, such that Akaike weights (wi) decline from 0.39 to 0.28 across these three models, and evidence ratios reaffirm the lack of a single dominant model (Table 1). Similarly, the variation explained by these models ranges from 20 % (Type I) to 25% (Type III).
We reject a Type I functional response, both because the data suggest a clear threshold in per capita consumption (Figure 5) and because handling time, stomach capacity, and digestive physiology all would be expected to limit consumption at some level of prey availability. Our data appear insufficient to distinguish between a Type II and Type III model, although Occam’s Razor would encourage acceptance of the less complex Type II model. As such, we interpret these data to suggest a weak Type II functional response with an apparent asymptote above a population density of about 100 individuals (Figure 5).
Influence of non-native lagomorphs. Non-native lagomorphs were consumed by Culpeos throughout our study, but their representation in scat increased modestly in 1997 and then markedly in 2003 (Figure 6). Consumption subsequently declined but then increased again after mid-2011. Overall, however, relative to small mammals, lagomorphs remain minor diet elements, and summary statistics (diet breadth, H’, evenness) do not vary notably when lagomorphs are incorporated (data not shown). Although data remain limited, a regression of the abundance of lagomorphs in Culpeo scat on five years of lagomorph pellet count data (an estimate of lagomorph abundance) are suggestive of a positive association, but this remains nonsignificant (F1,9 = 3.24, P = 0.11, R2 = 0.26; Figure 6).
Discussion
Culpeos are facultative specialists. In the face of extensive variation in availability of prey, Culpeos maintain a diverse diet, but small mammals remained the foundation of this diet over the 23-years of this study. Moreover, Culpeos are highly selective, consuming Abrocoma bennettii disproportionately in all months of study, and varying in the extent of preference for, or avoidance of, Octodon degus, relative to their contribution to local assemblages. Reflecting earlier work at this site, Culpeo appear to avoid Phyllotis, but unlike earlier studies they also avoided A. olivacea and the sole marsupial at our site, Thylamys. Phyllotis and Thylamys are largely nocturnal, and A. olivacea ranges from nocturnal to crepuscular to even diurnal, likely as a function of abundance and associated resource availability. Culpeos are labile foragers and are capable of foraging at any hour of the day, evidently as a function of the availability of favored prey (Iriarte et al. 1989b; Johnson and Franklin 1994) as well as human persecution. In Argentina, highland Peru, the Chilean desert and Magallanes, Culpeos are almost completely nocturnal (Crespo and de Carlo 1963; Crespo 1975; Johnson 1992; Novaro 1997a, b). This contrasts with their largely diurnal activity patterns in north central Chile (Jiménez 1993; Salvatori et al. 1999), where Culpeos are protected. Hence the apparent avoidance of Phyllotis, A. olivacea, and Thylamys may reflect the largely nocturnal habits of these species at Fray Jorge. Additionally, Thylamys is an insectivorous / frugivorous marsupial and may simply be unpalatable; they have a distinct odor (frequently notable to field workers while animals are still in a closed trap) and when handled they often defecate very moist and somewhat noxious scat. Finally, it is worth noting that all three of these species are relatively small and would provide limited resources on an individual basis.
Avoidance of nocturnal species, though, contrasts with the clear preference for Abrocoma, which is generally nocturnal in our study area. However, this species also is the largest rodent in our study area, and while relatively uncommon on our study grids, our data on availability of Abrocoma likely reflect underestimates as this species appears to be more abundant in more mesic aguada habitats located near the study plots (Milstead et al. 2007, DAK pers. obs.). Further work is needed to better characterize both the spatial patterns of abundance of this species, and its temporal patterns of activity.
We do not fully understand the mixed responses to Degus, which are diurnal, highly social, relatively abundant at Fray Jorge, and among the larger prey items available there. Degus generally are thought to be a favored prey of Culpeos (Meserve et al. 1987; Iriarte et al. 1989b), and this species is the second most common small mammal in Culpeo scat (Figure 2c, e). At our study site, however, we found no evidence that Culpeos preferentially fed on Degus more than expected by random chance, with no apparent relation to prey abundance except at the very highest densities (Figure 3h).
Neither Oligoryzomys nor A. longipilis appear to be important elements of Culpeo diets at Fray Jorge, and neither species was selected or avoided by Culpeos in our study; as noted earlier, however, both of these species are more characteristic of other habitats at Fray Jorge (Milstead et al. 2007; Meserve et al. 2016), and so our estimates of availability may be biased.
As a generality, Culpeos are opportunistic and solitary predators, but they exhibit a highly variable diet, both spatially and temporally (Jiménez and Novaro 2004; Guntiñas et al. 2017; Guntiñas et al. 2021; Lozano et al. 2024). Principal prey range from wild ungulates to domestic sheep, native and introduced lagomorphs, and small mammals; generally, other vertebrates (lizards, birds) and insects make up a small component of Culpeo diet, although insects may be locally or seasonally important (Correa and Roa 2005). In Argentine Patagonia, Culpeos selected among rodent species for those that may be more vulnerable (Corley et al. 1995). Culpeos in central Chile select the largest small mammals available (Meserve et al. 1987; Iriarte et al. 1989b; Jaksic et al. 1993). Culpeos have been considered trophically restricted (e. g., strict carnivores, near-insectivore, or generally frugivorous—Iriarte et al. 1989b; Cornejo and Jimenez 2001; Guzmán-Sandoval et al. 2007, respectively), or as a highly plastic predator capable of capitalizing on a broad range of resources (Jaksic et al. 1993; Castro et al. 1994; Johnson and Franklin 1994). Integrating across studies, Guntiñas et al. (2017; see also Guntiñas et al. 2021; Lozano et al. 2024) have argued that Culpeos are facultative specialists, presumably with local preferences reflecting optimal foraging strategies. Ours are the first data to support such a characterization over an extended period.
These foraging characteristics resemble those of many other medium-sized canids. Coyotes (Canis latrans) are opportunistic, generalist predators that eat a variety of food items, typically consuming items in relation to changes in availability. Coyotes may adjust activity patterns seasonally or in response to human disturbance or persecution (Kitchen et al. 2000), as observed for Culpeos. Similarly, Black-backed and Side-striped jackals (Lupulella adusta) are opportunistic and omnivorous predators and scavengers whose diet varies according to food availability (Atkinson et al. 2002; Loveridge and Macdonald 2002, 2003; Sillero-Zubiri et al. 2004; Skinner and Chimimba 2005; Sillero-Zubiri 2009). Red Fox (Vulpes vulpes) are adaptable and opportunistic omnivores, with a diet ranging from invertebrates (e. g., earthworms and beetles) to mammals and birds (including game birds), and fruit. They also scavenge in rural areas. Also similar to Culpeos, Red Fox forage mainly during nocturnal and crepuscular periods, although they are more diurnal where undisturbed (Macdonald and Reynolds 2004).
Culpeo foxes exhibit both numerical and functional responses. Culpeo foxes occur in a wide variety of habitats over a large geographic range (see Introduction). Such a large range would suggest trophic flexibility, and as outlined above, this species is known to be a generalist forager and to capitalize on diverse foods, ranging from fruits and seeds to insects, small vertebrates, and carrion. In general, however, Culpeos favor small mammals (Lozano et al. 2024), and within this group they often forage selectively, favoring some species over others (e. g., Jaksic et al. 1992, 1993, this study), and while other studies have demonstrated numerical responses to variation in key prey species, this is the first to document functional responses. At Las Chinchillas National Reserve, located in the Andean foothills less than 100 km from Fray Jorge, Jaksic et al. (1992) showed that the diet of Culpeos and Chillas continued to emphasize A. bennettii and O. degus through an irruption of A. olivacea and P. darwini; foxes there consumed the latter two species in proportion to availability. In earlier work at Fray Jorge, Culpeos “showed strong prey preferences for some mammalian species [most notably, Abrocoma], regardless of their abundance in the field, and thus failed to display functional responses” (Jaksic et al. 1993:305). After an irruption of small mammals following ENSO-associated rains in 1991, Culpeos at Fray Jorge “appeared to select O. degus” (Jaksic et al. 1997:346). These studies were based on limited time series, however, and discrepancies with results presented here (using over two decades of data) support a caveat by Jaksic et al. (1996:252) “that short-term studies of species assemblages be used cautiously.”
Numerical responses to variation in small mammal abundance are clear from our data (Figures 1 and, 4). What is novel here, however, is the demonstration of a Type II functional response as well (Table 1, Figure 5). Culpeos show a clear increase in per capita consumption as prey population sizes increase (Table 1, Figure 5). Further work will be needed to clarify how this response varies across prey species, but data from both Fray Jorge and Las Chinchillas suggest that Culpeos are likely to select Abrocoma and O. degus, although data presented here suggest that the latter may be abundant in the diet of Culpeos merely because they are abundant in the landscape. As noted above, these are two of the largest species of rodents in the region, and therefore should provide the greatest energetic value.
A generalist predator would be expected to exhibit functional responses to variation in other prey as well (e. g., non-mammalian prey). Our data support such a conjecture, as the proportion of non-mammalian prey in some years (e. g., 1990 to 91, 1994 to 96, 1999 to 2002, 2004, 2006 to 11) increases markedly (Figure 2b), and even seed consumption (Figure 2a) varies notably over time. These functional responses are more difficult to characterize, however, as we lack data on availability for these food items, and diagnostic characteristics often do not appear in scat. However, it appears that the generalist Culpeo, like many other small canids, readily diversifies its diet under in the face of variation in food availability.
The role of spatial heterogeneity. While our research on small mammal assemblages (and hence our estimates of prey availability) is restricted largely to thorn scrub habitat, Fray Jorge includes other distinct habitats as well, and these appear to serve as source habitats for some small mammal species (Milstead et al. 2007). Perhaps most notably, moist habitat occurs where the water table remains high enough to support mesic vegetation (e. g., aguadas, which occur at the bottom of valleys), and coastal fog deposits sufficient moisture on higher elevations of coastal ranges (e. g., the Altos de Talinay) to support patches of forest reminiscent of Valdivian rainforest of south-central Chile (Villagrán et al. 2004, Squeo et al. 2016). The former habitat appears to be key for species such as O. longicaudatus and likely Abrocoma, whereas the latter is a source for both A. longipilis and O. longicaudatus, both of which “spill over” to our study plots in thorn scrub during wet years when source populations experience growth (Milstead et al. 2007).
A second species of Octodon also occurs in moister habitats in Fray Jorge and merits further consideration; O. lunatus is the second largest small mammal at Fray Jorge, so we assume it would be favored when available. We tallied 101 individuals of O. lunatus in scat collected during 25 of 46 seasons analyzed here (in contrast to 1,148 individuals of O. degus in 45 of 46 sessions), but we captured this species in our monthly trapping efforts only in 11 seasons (and never in abundance), presumably reflecting habitat preferences. Ancillary trapping in aguada habitats supports a hypothesis that O. lunatus is more prevalent here than in thorn scrub (Milstead et al. 2007), but given the lack of data on availability we have excluded this species from analysis here. Further efforts to characterize demographic patterns in these key habitats, and to tie these to predator behavior and foraging, would be highly instructive.
Are foxes turning to lagomorphs? Although lagomorphs have been present in Culpeo scat through most of our study, it is only since about 2003 that they have contributed substantially to scat composition (Figures 2 and 6). The increase in lagomorph remains in Culpeo scat in 2003 to '07 followed both a series of high rainfall years (2000 to '02) and a 2002 to '03 outbreak of distemper in Culpeos at Fray Jorge, so it is difficult to determine which of these influences led to the apparent increase in consumption of lagomorphs, although lagomorphs increased in prevalence at Las Chinchillas at about the same time (F. Jaksic pers. comm. to DAK, October 2016), suggesting that the elevated rainfall influenced lagomorph abundance regionally. We initiated surveys of lagomorph activity in 2008 (symbols in Figure 6a), and limited data since then are suggestive of a relationship between lagomorph abundance and their presence in fox scat, although additional data are needed to resolve this association. However, such a delayed response to the presence of invasive lagomorphs by Culpeos would reflect similar observations for Black-chested Buzzard Eagles (Geranoaetus melanoleucus—Pavez et al. 2010). We predict that Culpeos will increase their consumption of invasive lagomorphs, especially the more abundant Oryctolagus, during years of high rabbit abundance or low small mammal abundance.
More on the value of long-term data. In the first decade of this project, Degus were positively selected by Culpeos in 15 of 22 6-mo periods (Figure 3). It was during this period that Jaksic et al. (1993) reported that Culpeos exhibited strong preferences for certain prey, and these preferences did not vary with fluctuations in different prey species, thereby displaying no functional response. A slightly longer database allowed Jaksic et al. (1997) to document an apparent functional response, as Culpeos shifted from Abrocoma (which they generally favored) towards Degus as the latter increased in abundance.
These earlier analyses were undertaken well before an apparent change in climatic patterns (ca. 2001/02) that led to local dominance by Degus (Meserve et al. 2011, 2016). From about 2001 through the period reported here (a mega-drought—CR2 2015, Garreaud et al. 2020), Degus comprised 60 % or more of the small mammal biomass at Fray Jorge; oddly, throughout this time (e. g., since 2001NR in Figure 3g) Degus were negatively selected in all but four periods (e. g., 21 of 25 6-mo periods), representing an almost symmetrical pattern relative to that observed prior to this period, when Degus were consumed in excess of apparent availability. This change may reflect the apparent increase in consumption of lagomorphs (compare Octodon selection in Figure 3g against the presence of lagomorphs in scats in Figures 2 and 6). Regardless, it is presumably the increased resolution provided by the unusually long-term window of observation here that has allowed us to detect such a clear functional response to changes in small mammal numbers. Further work is needed to dissect this functional response among component small mammal species.
Acknowledgments
We thank Marjorie Matocq and Eileen Lacey for an invitation to contribute to this volume. Jim Patton has been a friend and mentor to more mammalogists than he may appreciate, but we believe we speak for many in expressing our profound and perennial appreciation for his contributions to the field, and for his extreme generosity to others. We wish to dedicate this effort to the memory of two dear friends and colleagues, Brian K. Lang and Julio R. Gutiérrez. Brian Lang was one of the early leaders of our field crew at Fray Jorge, and he designed and implemented the olfactory lines that we continue to use to characterize Culpeo activity. As such, this aspect of predator monitoring would not exist had it not been for his initiative and early contributions. Julio Gutiérrez was one of the initial principal investigators responsible for establishing this long-term study (see Meserve 2016). Their passion, dedication, and invaluable insights have made a lasting impact on this work. They will be greatly missed, both as collaborators and as friends. We thank B. Cypher for suggesting the method employed here for lagomorph olfactory surveys. B. Cypher and two anonymous reviewers also provided valuable review of the manuscript; we believe that the final product is greatly improved because of their honest critiques. This project has been funded by both the National Science Foundation (most recently, DEB 2025816 to DAK, Seth Newsome, and Justin Yeakel) and CONICYT (most recently, CONICYT/ FONDECYT 1160026 to JRG). We gratefully acknowledge assistance from both CEAZA (Centro de Estudios Ambientales de Zonas Áridas, Univ. La Serena, Chile) and IEB (Instituto de Ecología y Biodiversidad; P. Univ. Católica de Chile, Santiago). Additionally, DAK acknowledges the USDA National Institute of Food and Agriculture, Hatch project CA-D-WFB-6126-H. Finally, it is only with the continued collaborative arrangement that we have established with the Chilean park service, CONAF, that this effort is possible; we thank them for their support and cooperation.
Literature cited
Acosta Jamett, G. A. 2010. The role of domestic dogs in diseases of significance to human and wildlife health in central Chile. Ph.D. dissertation, University of Edinburgh. Edinburgh, United Kingdom.
Aleksandra, P., and C. Dusko. 2015. Seasonal variation in diet of the golden jackal (Canis aureus) in Serbia. Mammal Research 60:309-317.
Angerbjorn, A., M. Tannerfeldt, and S. Erlinge. 1999. Predator-prey relationships: Arctic foxes and lemmings. Journal of Animal Ecology 68:34-49.
Armas, C., J. R., et al. 2016a. Twenty-five years of research in the north-central Chilean semiarid zone: the Fray Jorge Long-Term Socio-Ecological Research (LTSER) site and Norte Chico. Journal of Arid Environments 126:1-6.
Armas, C., J. R., et al. 2016b. Twenty-five years of research in the north-central Chilean arid zone: the Fray Jorge Long-Term Socio-Ecological Research (LTSER) site and Norte Chico. Journal of Arid Environments 129:1-88.
Atkinson, R. P. D., D. W. MacDonald, and R. Kamizola. 2002. Dietary opportunism in side-striped jackals Canis adustus Sundevall. Journal of Zoology 257:129-139.
Camus, P., S. Castro, and F. Jaksic. 2008. El conejo Europeo en Chile: historia de una invasión biológia. Historia 41:305-339.
Castro, S. A., et al. 1994. Frugivoría y dispersión de semillas de pimiento (Schinus molle) por el zorro culpeo (Pseudalopex culpaeus) en el Parque Nacional Fray Jorge (IV Región, Chile). Revista Chilena de Historia Natural 67:169-176.
Corley, J. C., et al. 1995. Selection of cricetine prey by the culpeo fox in Patagonia: a differential prey vulnerability hypothesis. Mammalia 59:315-325.
Cornejo, A., and P. Jimenez. 2001. Dieta del Zorro Andino Pseudalopex culpaeus (Canidae) en el Matorral Desértico del Sur del Perú. Revista de Ecología Latinoamericana 8:1-9.
Correa, P., and A. Roa. 2005. Nutritional relations between Oncifelis guigna, Lycalopex culpaeus, Lycalopex griseus and Tyto alba in a fragmented ambience of the central area of Chile. Mastozoologia Neotropical 12:57-60.
CR2. 2015. [Center for Climate and Resilience Research] Report to the nation: the Central Chile mega-drought. Technical report. 30 pp Santiago (Chile). https://www.cr2.cl/megasequia (Accessed 21 August 2024).
Crespo, J. A. 1975. Ecology of the pampas grey fox and the large fox (culpeo). Pp. 179-190, in The wild canids (M. W. Fox, ed.). Van Nostrand Reinhold Company. New York, USA.
Crespo, J. A., and J. M. de Carlo. 1963. Estudio ecológico de una población de zorros colorados, Dusicyon culpaeus culpaeus (Molina) en el oeste de la provincia de Neuquén. Revista del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia,” Ecología 1:1-55.
Denny, M. 2014. Buzz Holling and the functional response. Bulletin of the Ecological Society of America 95:200-202.
Elmhagen, B., et al. 2000. The Arctic fox (Alopex lagopus): an opportunistic specialist. Journal of Zoology 251:139-149.
Etheredge, C. R., et al. 2015. Local-scale difference of coyote food habits on two South Carolina islands. Southeastern Naturalist 14:281-292.
Feinsinger, P., E. E. Spears, and R. W. Poole. 1981. A simple measure of niche breadth. Ecology 62:27-32.
Fulk, G. W. 1975. Population ecology of rodents in the semiarid shrublands of Chile. Occasional Papers, The Museum, Texas Tech University 33:1-40.
Gantchoff, M. G., and J. L. Belant. 2016. Patterns of coexistence between two mesocarnivores in northern Patagonia in the presence of invasive hares and anthropogenic disturbance. Austral Ecology 41:97-105.
Garreaud, R. D., et al. 2020. The Central Chile mega drought (2010–2018): a climate dynamics perspective. International Journal of Climatology 40:421-439.
Gittleman, J. L. 1985. Carnivore body size: ecological and taxonomic correlates. Oecologia 67:540-554.
Guntiñas, M., et al. 2021. Ecology of the culpeo (Lycalopex culpaeus): a synthesis of existing knowledge. Hystrix-Italian Journal of Mammalogy 32:5-17.
Guntiñas, M., et al. 2017. Feeding ecology of the culpeo in southern Ecuador: wild ungulates being the main prey. Contributions to Zoology 86:169-180.
Gutiérrez, J. R., et al. 1997. Effects of small mammals and vertebrate predators on vegetation in the Chilean semiarid zone. Oecologia 109:398-406.
Gutiérrez, J. R., et al. 1993. Structure and dynamics of vegetation in a Chilean arid thornscrub community. Acta Oecologica 14:271-285.
Gutiérrez, J. R., P. L. Meserve, and D. A. Kelt. 2004. Estructura dinámica de la vegetación del ecosistema semiárido del Parque Nacional Bosque Fray Jorge entre 1989 y 2002. Pp. 115-134, in Historia Natural del Parque Nacional Fray Jorge, Región de Coquimbo, Chile (Squeo, F. A., J. R. Gutiérrez, and I. R. Hernández, eds.). Ediciones Universidad de La Serena. La Serena, Chile.
Gutiérrez, J. R., et al. 2010. Long-term research in Bosque Fray Jorge National Park: twenty years studying the role of biotic and abiotic factors in a Chilean semiarid scrubland. Revista Chilena de Historia Natural 83:69-98.
Guzmán-Sandoval, J., W. Sielfeld, and M. Ferrú. 2007. Dieta de Lycalopex culpaeus (Mammalia: Canidae) en el extremo norte de Chile (Region de Tarapaca). Gayana 71:1-7.
Holling, C. S. 1959. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Canadian Entomologist 91:293-320.
Humphries, B. D., T. Ramesh, and C. T. Downs. 2016. Diet of black-backed jackals (Canis mesomelas) on farmlands in the KwaZulu-Natal Midlands, South Africa. Mammalia 80:405-412.
Iriarte, A., and F. Jaksic. 2012. Los carnívoros de Chile. Ediciones Flora y Fauna and CASEB. P. Universidad Católica de Chile, Santiago, Chile.
Iriarte, J. A., L. C. Contreras, and F. M. Jaksic. 1989a. A long-term study of a small mammal assemblage in the central Chilean matorral. Journal of Mammalogy 70:79-87.
Iriarte, J. A., et al. 1989b. Small mammal availability and consumption by the fox, Dusicyon culpaeus, in central Chilean scrublands. Journal of Mammalogy 70:641-645.
Jaksic, F., and S. A. Castro. 2014. Invasiones biológicas en Chile: causas globales e impactos locales. Ediciones Universidad Católica de Chile. Santiago, Chile.
Jaksic, F. J., et al. 1993. The components of predation on small mammals in semiarid Chile: preliminary results. Revista Chilena de Historia Natural 66:305-321.
Jaksic, F. M. 1998. Ecología de los vertebrados de Chile. 2nd ed. Ediciones Universidad Católica de Chile. Santiago, Chile.
Jaksic, F. M., P. Feinsinger, and J. E. Jimenez. 1996. Ecological redundancy and long-term dynamics of vertebrate predators in semiarid Chile. Conservation Biology 10:252-262.
Jaksic, F. M., et al. 1992. Numerical and functional responses of predators to a long-term decline in mammalian prey at a semi-arid Neotropical site. Oecologia 89:90-101.
Jaksic, F. M., et al. 1997. A long-term study of vertebrate predator responses to an El Nino (ENSO) disturbance in western South America. Oikos 78:341-354.
Jaksic, F. M., and J. L. Yáñez. 1983. Rabbit and fox introductions in Tierra del Fuego: history and assessment of the attempts at biological control of the rabbit infestation. Biological Conservation 26:367-374.
Jiménez, J. E. 1993. Comparative ecology of Dusicyon foxes at the Chinchilla National Reserve in northcentral Chile. Master's Thesis, University of Florida. Gainesville, Florida, USA.
Jiménez, J. E., P. Feinsinger, and F. M. Jaksic. 1992. Spatiotemporal patterns of an irruption and decline of small mammals in northcentral Chile. Journal of Mammalogy 73:356-364.
Jiménez, J. E., and A. J. Novaro. 2004. Culpeo Pseudalopex culpaeus (Molina, 1782). Pp. 44-49, in Canids: foxes, wolves, jackals and dogs. Status survey and conservation action plan (Sillero-Zubiri, C., M. Hoffmann, and D. W. Macdonald, eds.). IUCN/SSC Canid Specialist Group. Gland, Switzerland and Cambridge, UK.. Gland, Switzerland and Cambridge, UK.
Johnson, W. E. 1992. Comparative ecology of two sympatric South American foxes Dusicyon griseus and D. culpaeus. Ph.D. dissertation, Iowa State University. Ames, Iowa, USA.
Johnson, W. E., and W. L. Franklin. 1994. Role of body size in the diets of sympatric gray and culpeo foxes. Journal of Mammalogy 75:163-174.
Kelt, D. A., et al. 2004a. Seed predation by birds and small mammals in semiarid Chile. Oikos 104:133-141.
Kelt, D. A., P. L. Meserve, and J. R. Gutiérrez. 2004b. Seed removal by small mammals, birds and ants in semi-arid Chile, and comparison with other systems. Journal of Biogeography 31:931-942.
Kelt, D. A., et al. 2013. Long-term monitoring of mammals in the face of biotic and abiotic influences at a semiarid site in north-central Chile. Ecology 94:977.
Kelt, D. A., et al. 2004c. Foraging ecology of small mammals in semiarid Chile: the interplay of biotic and abiotic effects. Ecology 85:383-397.
Kitchen, A. M., E. M. Gese, and E. R. Schauster. 2000. Changes in coyote activity patterns due to reduced exposure to human persecution. Canadian Journal of Zoology 78:853-857.
Laake, J. L. 2013. RMark: An R Interface for Analysis of Capture-Recapture Data with MARK. AFSC Processed Rep 2013-01, 25p. (available at https://cran.r-project.org/web/packages/RMark/index.html).
Loveridge, A. J., and D. W. Macdonald. 2002. Habitat ecology of two sympatric species of jackals in Zimbabwe. Journal of Mammalogy 83:599-607.
Loveridge, A. J., and D. W. MacDonald. 2003. Niche separation in sympatric jackals (Canis mesomelas and Canis adustus). Journal of Zoology 259:143-153.
Lozano, J., et al. 2024. Diversity and biogeographical patterns in the diet of the culpeo in South America. Ecology and Evolution 14:e70176.
Lucherini, M. 2016. Lycalopex griseus (errata version published in 2017)in The IUCN Red List of Threatened Species 2016: e.T6927A111975602. http://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T6927A86440397.en. Downloaded on 22 August 2024.
Lucherini, M., and E. M. Luengos Vidal. 2008. Lycalopex gymnocercus (Carnivora: Canidae). Mammalian Species 820:1-9.
Macdonald, D. W., and J. C. Reynolds. 2004. Red fox Vulpes vulpes Linnaeus, 1759. Pp. 129-136, in Canids: foxes, wolves, jackals and dogs. Status survey and conservation action plan (Sillero-Zubiri, C., M. Hoffmann, and D. W. Macdonald, eds.). IUCN/SSC Canid Specialist Group. Gland, Switzerland and Cambridge, UK.. Gland, Switzerland and Cambridge, UK.
Magurran, A. E. 1988. Ecological diversity and its measurement. Princeton University Press. Princeton, New Jersey.
Marquet, P. A., et al. 1993. Food habits of Pseudalopex foxes in the Atacama desert, pre-Andean ranges, and the high-Andean plateau of northernmost Chile. Mammalia 57:130-135.
Martínez, D. R., J. R. Rau, and F. M. Jaksic. 1993. Respuesta numérica y selectividad dietaria de zorros (Pseudalopex spp.) ante una reducción de sus presas en el norte de Chile. Revista Chilena de Historia Natural 66:195-202.
Meserve, P. L. 1981a. Trophic relationships among small mammals in a Chilean semiarid thorn scrub community. Journal of Mammalogy 62:304-314.
Meserve, P. L. 1981b. Resource partitioning in a Chilean semi-arid small mammal community. Journal of Animal Ecology 50:745-757.
Meserve, P. L. 2016. Genesis, evolution, and future of a long-term study of small mammals in South America. Mastozoologia Neotropical 23:11-16.
Meserve, P. L., et al. 1993. Role of biotic interactions in a semiarid scrub community in north-central Chile: a long term ecological experiment. Revista Chilena de Historia Natural 66:225-241.
Meserve, P. L., et al. 1996. Role of biotic interactions in a small mammal assemblage in semiarid Chile. Ecology 77:133-148.
Meserve, P. L., et al. 2016. Biotic interactions and community dynamics in the semiarid thorn scrub of Bosque Fray Jorge National Park, north-central Chile: a paradigm revisited. Journal of Arid Environments 126:81-88.
Meserve, P. L., et al. 2003. Thirteen years of shifting top-down and bottom-up control. BioScience 53:633-646.
Meserve, P. L., et al. 2011. Global climate change and small mammal populations in north-central Chile. Journal of Mammalogy 92:1223-1235.
Meserve, P. L., W. B. Milstead, and J. R. Gutiérrez. 2001. Results of a food addition experiment in a north-central Chile small mammal assemblage: evidence for the role of "bottom-up" factors. Oikos 94:548-556.
Meserve, P. L., E. J. Shadrick, and D. A. Kelt. 1987. Diets and selectivity of two Chilean predators in the northern semi-arid zone. Revista Chilena de Historia Natural 60:93-100.
Meserve, P. L. et al. 1995. Heterogeneous responses of small mammals to an El Niño Southern Oscillation event in northcentral semiarid Chile and the importance of ecological scale. Journal of Mammalogy 76:580-595.
Milstead, W. B., et al. 2007. Spatial ecology of small mammals in north-central Chile: role of precipitation and refuges. Journal of Mammalogy 88:1532-1538.
Moreira-Arce, D., et al. 2015. Native forest replacement by exotic plantations triggers changes in prey selection of mesocarnivores. Biological Conservation 192:258-267.
Moreira, R., and M. Stutzin. 2005. Estudio de la mortalidad de zorros en la IV Región. Boletín Veterinario Oficial 3:1-8 (online at http://www2.sag.gob.cl/Pecuaria/bvo/marzo_mayo_2005/articulos/mortalidad_zorros_IV_region.pdf; accessed 15 December 2016).
Muñoz-Pedreros, A., J. Yáñez Valenzuela (eds.). 2009. Mamíferos de Chile, segunda edicion. CEA Ediciones, Santiago, Chile.
Murray, D. L., et al. 2002. Estimating low-density snowshoe hare populations using fecal pellet counts. Canadian Journal of Zoology 80:771-781.
Novaro, A. J. 1997a. Source-sink dynamics induced by hunting: case study of culpeo foxes on rangelands in Patagonia, Argentina. Dissertation, University of Florida. Ph.D. dissertation, Gainesville, Florida, USA.
Novaro, A. J. 1997b. Pseudalopex culpaeus. Mammalian Species 558:1-8.
O'Mahony, D., et al. 1999. Fox predation on cyclic field vole populations in Britain. Ecography 22:575-581.
Osgood, W. H. 1943. The mammals of Chile. Field Museum of Natural History, Zoological Series 30:1-268.
Palacios, R., R. Susan Walker, and A. J. Novaro. 2012. Differences in diet and trophic interactions of Patagonian carnivores between areas with mostly native or exotic prey. Mammalian Biology 77:183-189.
Palomares, F. 2001. Comparison of 3 methods to estimate rabbit abundance in a Mediterranean environment. Wildlife Society Bulletin 29:578-585.
Pavez, E. F., G. A. Lobos, and F. M. Jaksic. 2010. Long-term changes in landscape and in small mammal and raptor assemblages in central Chile. Revista Chilena de Historia Natural 83:99-111.
Pia, M. V., M. S. López, and A. J. Novaro. 2003. Effects of livestock on the feeding ecology of endemic culpeo foxes (Pseudalopex culpaeus smithersi) in central Argentina. Revista Chilena de Historia Natural 76:313-321.
Previtali, M. A., et al. 2009. Population dynamics of two sympatric rodents in a variable environment: rainfall, resource availability, and predation. Ecology 90:1996-2006.
R Core Team. 2024. R: a language and environment for statistical computing. Vienna, Austria https://www.R-project.org/: R Foundation for Statistical Computing.
Rubio, A. V., R. Alvarado, and C. Bonacic. 2013. Introduced European rabbit as main prey of the native carnivore culpeo fox (Lycalopex culpaeus) in disturbed ecosystems of central Chile. Studies on Neotropical Fauna and Environment 48:89-94.
Salvatori, V., et al. 1999. Spatial organization, activity, and social interactions of culpeo foxes (Pseudalopex culpaeus) in north-central Chile. Journal of Mammalogy 80:980-985.
Schamberger, M., and G. Fulk. 1974. Mamíferos del Parque Nacional Fray Jorge. Idesia 3:167-180.
Scheffer, M. 2009. Critical transitions in nature and society. Princeton University Press. Princeton, New Jersey.
Schwarz, C. J., and A. N. Arnason. 1996. A general methodology for the analysis of capture-recapture experiments in open populations. Biometrics 52:860-873.
Sikes, R. S., and the Animal Care and Use Committee of the American Society of Mammalogists. 2016. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy 97:663-688.
Sillero-Zubiri, C. 2009. Family Canidae (dogs). Pp. 352-446, in Handbook of the mammals of the world. Vol. 1. Carnivores (Wilson, D. E., and R. A. Mittermeier, eds.). Lynx Edicions. Barcelona, Spain.
Sillero-Zubiri, C., M. Hoffmann, and D. W. Macdonald. 2004. Canids: foxes, wolves, jackals, and dogs. Status Survey and Conservation Action Plan. IUCN/SSC Canid Specialist Group. Switzerland and Cambridge, U.K. Gland.
Skinner, J. D., and C. T. Chimimba. 2005. The mammals of the southern African subregion. 3rd ed. Cambridge University Press. Cambridge, UK.
Squeo, F. A., J. R. Gutiérrez, and I. R. Hernández. 2004. Historia Natural del Parque Nacional Bosque Fray Jorge. Ediciones Universidad de La Serena. La Serena, Chile.
Squeo, F. A., et al. 2016. Vegetation of Bosque Fray Jorge National Park and its surrounding matrix in the Coastal Desert of north-central Chile. Journal of Arid Environments 126:12-22.
Villagrán, C., et al. 2004. El enigmático origen del bosque relicto de Fray Jorge. Pp. 3-43, in Historia Natural del Parque Nacional Bosque Fray Jorge (Squeo, F. A., J. R. Gutiérrez, and I. R. Hernández, eds.). Ediciones Universidad de La Serena. La Serena, Chile.
Walker, R. S., et al. 2007. Diets of three species of Andean carnivores in high-altitude deserts of Argentina. Journal of Mammalogy 88:519-525.
White, G. C., and K. P. Burnham. 1999. Program MARK: survival estimation from populations of marked animals. Bird Study 46 (Supplement):S120-S139.
Williams, B. K., J. D. Nichols, and M. J. Conroy. 2002. Analysis and management of animal populations: modeling, estimation, and decision making. Academic Press. San Diego, California.
Yunger, J. A., P. L. Meserve, and J. R. Gutiérrez. 2002. Small-mammal foraging behavior: mechanisms for coexistence and implication for population dynamics. Ecological Monographs 72:561-577.
Zapata, S. C., et al. 2005. Food habits and resource partitioning between grey and culpeo foxes in southeastern Argentine Patagonia. Studies on Neotropical Fauna and Environment 40:97-103.
Associated editors: Marjorie Matocq and Eileen Lacey
Submitted: September 7, 2024; Reviewed: September 29, 2024
Accepted: November 4, 2024; Published on line: January 31, 2025
Supplementary material
https://mastozoologiamexicana.com/therya/index.php/THERYA/article/view/6150/1474

Figure 1. Estimated population sizes for small mammals on four control trapping grids over 23 years at Fray Jorge. Triangles and dashed lines indicate the number of olfactory stations visited by Culpeo (Lycalopex culpaeus) in 6-mo periods from December 1990 through August 2008 (except the first bar which represents only three months of observation (Dec. to Feb.), and NR1994 which is missing data for June 1994). NR = period of non-reproductive activity (e. g., NR1991 = Mar. to Aug. 1991); R = period of small mammal reproduction (e. g., R1991 = Sep. 1991 to Feb. 1992). Periods of high rainfall are indicated with gray shading.
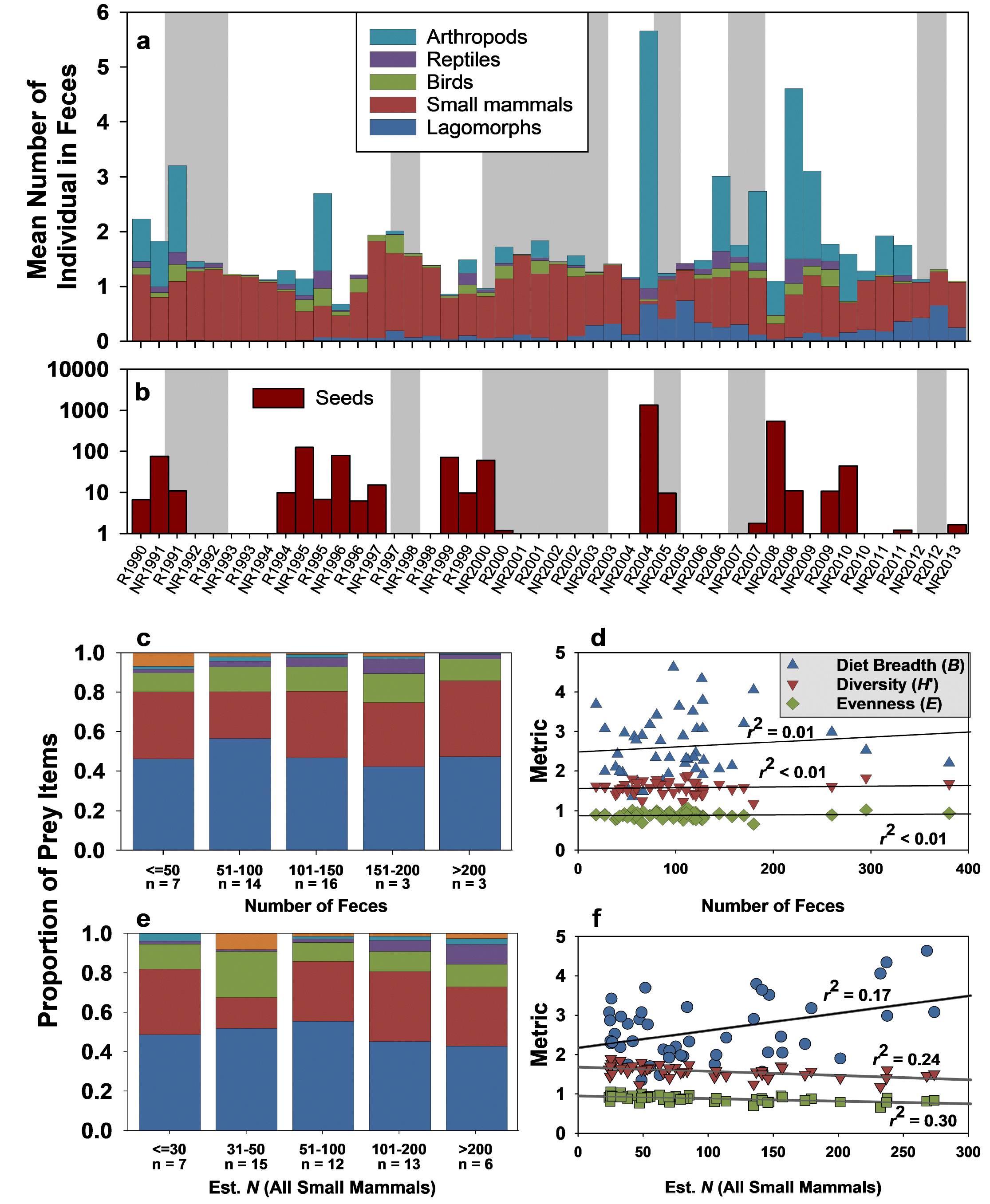
Figure 2. General dietary composition for Culpeo (Lycalopex culpaeus) across 23 years at Fray Jorge, Chile. (a) Seeds are present in the diet sporadically and in highly variable numbers (note the log axis). (b) Culpeo emphasize small mammals in their diet even as small mammal numbers vary greatly (cf. Figure 1). Lagomorphs have increased in representation since about 2002. Other taxa are consumed more opportunistically over time. The proportional composition of small mammal remains in Culpeo scat is invariant across (c, d) variation in number of scat sampled (as one metric of fox abundance or activity) or (e, f) the number of individual mammals in scat (as an index of small mammal population sizes). Panels d and f present metrics pertaining to panels c and e, respectively; linear regressions in d are non-significant (all P > 0.45), those in f are all significant (all P < 0.005). Panels c through f do not include lagomorphs.
Figure 3. Selectivity of seven species of small mammals in the diet of Culpeo (Lycalopex culpaeus) across 23 years at Fray Jorge, Chile. Black vertical bars present population estimates calculated over 6-mo periods, while green and red vertical bars represent selection and avoidance, respectively, of these species in the same periods. Scatterplots to the right show selectivities as a function of population estimates for each small mammal species; dashed gray line reflects zero selectivity, and values presented are the number of positive and negative selectivity values >1.5 and associated probabilities in a binomial test (see Supplementary material Table S4).
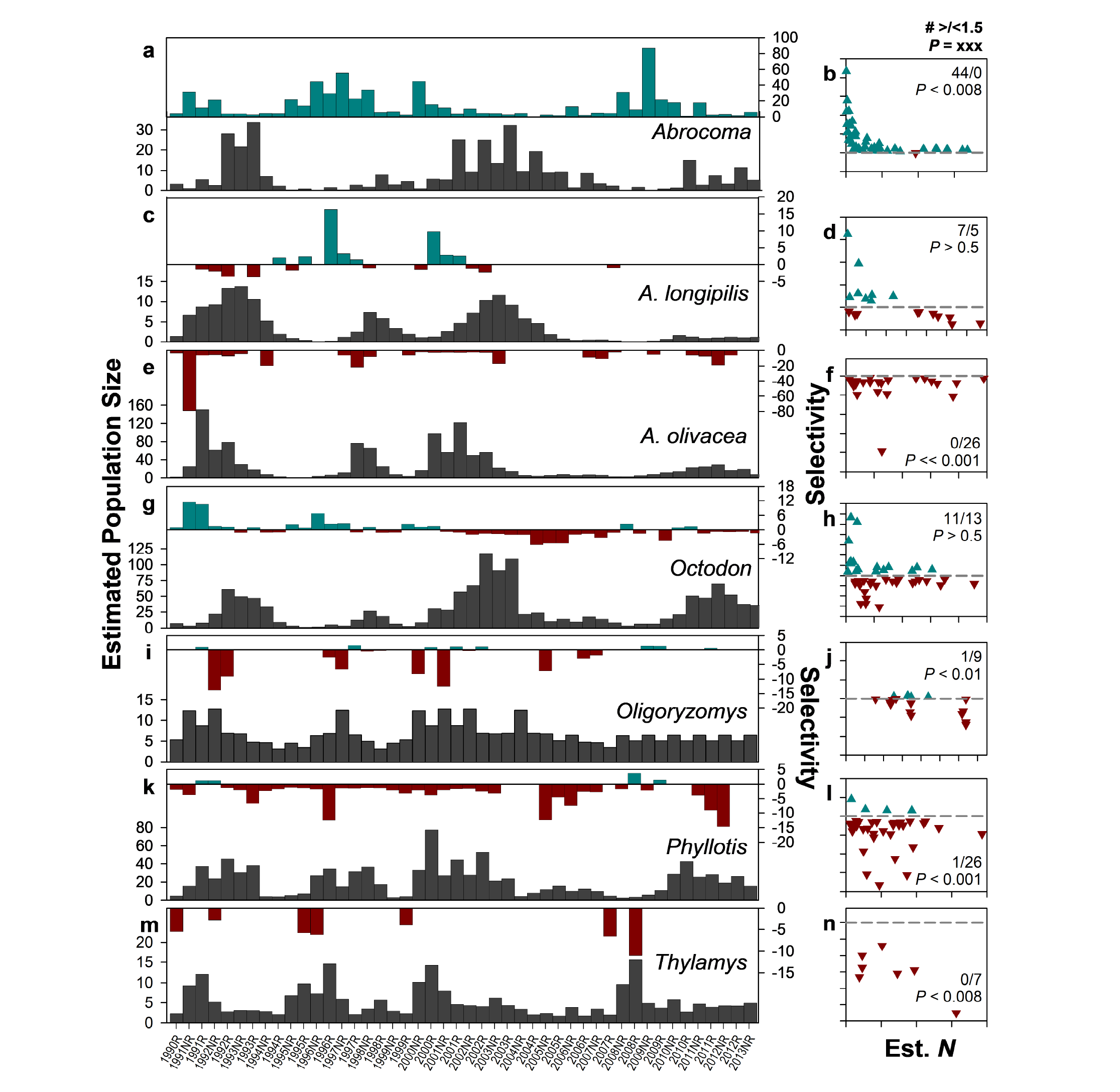
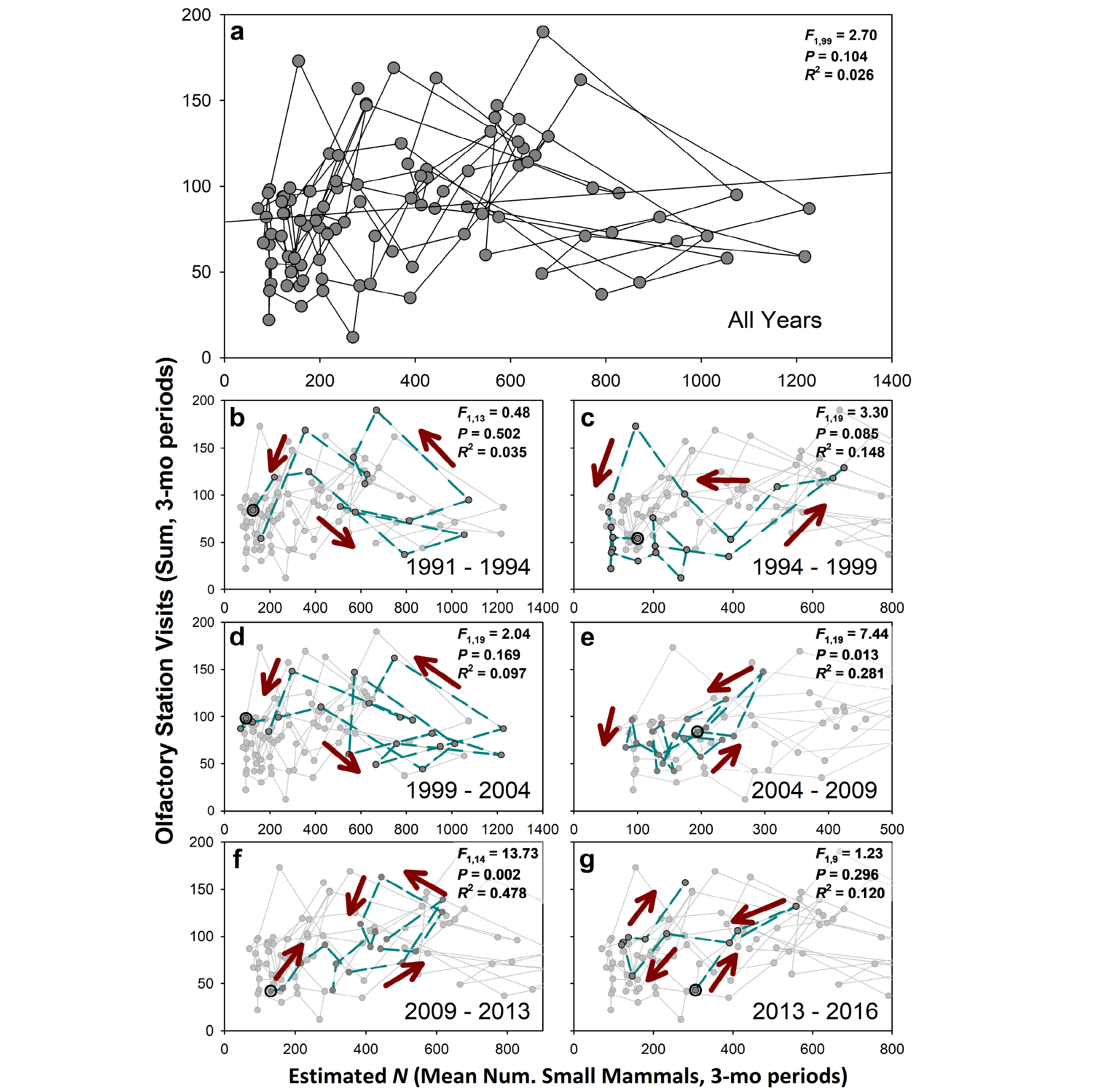
Figure 4. Numerical response of Culpeo (Lycalopex culpaeus) to temporal variation in small mammal populations across 23 years at Fray Jorge, Chile. The first panel presents all time periods (101 3-mo periods), while subsequent panels highlight separate multi-year cycles suggestive of prey tracking by Culpeo (e. g., variably counter-clockwise trajectories). Note that the x-axes in panels e through g have been adjusted to illustrate patterns during cycles with relatively low small mammal population. Red arrows illustrate the trajectory of data points (large circle indicates starting point for each panel). A similar analysis with 6-mo windows increases the fit to the data (linear regression, F1,44 = 10.62, P = 0.0022) but the explanatory power remains low (R2 = 0.19).
Table 1. Regression of consumption of small mammals by Culpeo (Lycalopex culpaeus) against estimated population size across all small mammals at Fray Jorge, Chile. Although we assume that foxes satiate (stomach size is finite so consumption should asymptote) we applied linear, polynomial, and sigmoidal regressions as estimates of Type I, Type II, and Type III functional responses, respectively. A null (intercept) model was employed for comparison against a lack of any functional response. All models were compared using Akaike’s Information Criterion (AICc) and Akaike weights (wi).
|
Order |
Model |
R2 |
AICc |
∆AICc |
wi |
|
Type I |
y = 0.517 + 2.07 × 10-3 (x) |
0.202 |
-106.637 |
0 |
0.389 |
|
Type II |
y = 0.379 + 5.3 × 10-3 (x) -1.24 × 10-5 (x2) |
0.237 |
-106.296 |
0.341 |
0.328 |
|
Type III |
y = 0.99 ⁄ (1 + e - (x - 62.17)/20.73) |
0.247 |
-105.997 |
0.641 |
0.283 |
|
Null |
y = 0.723 |
-52.570 |
54.067 |
7x10-13 |
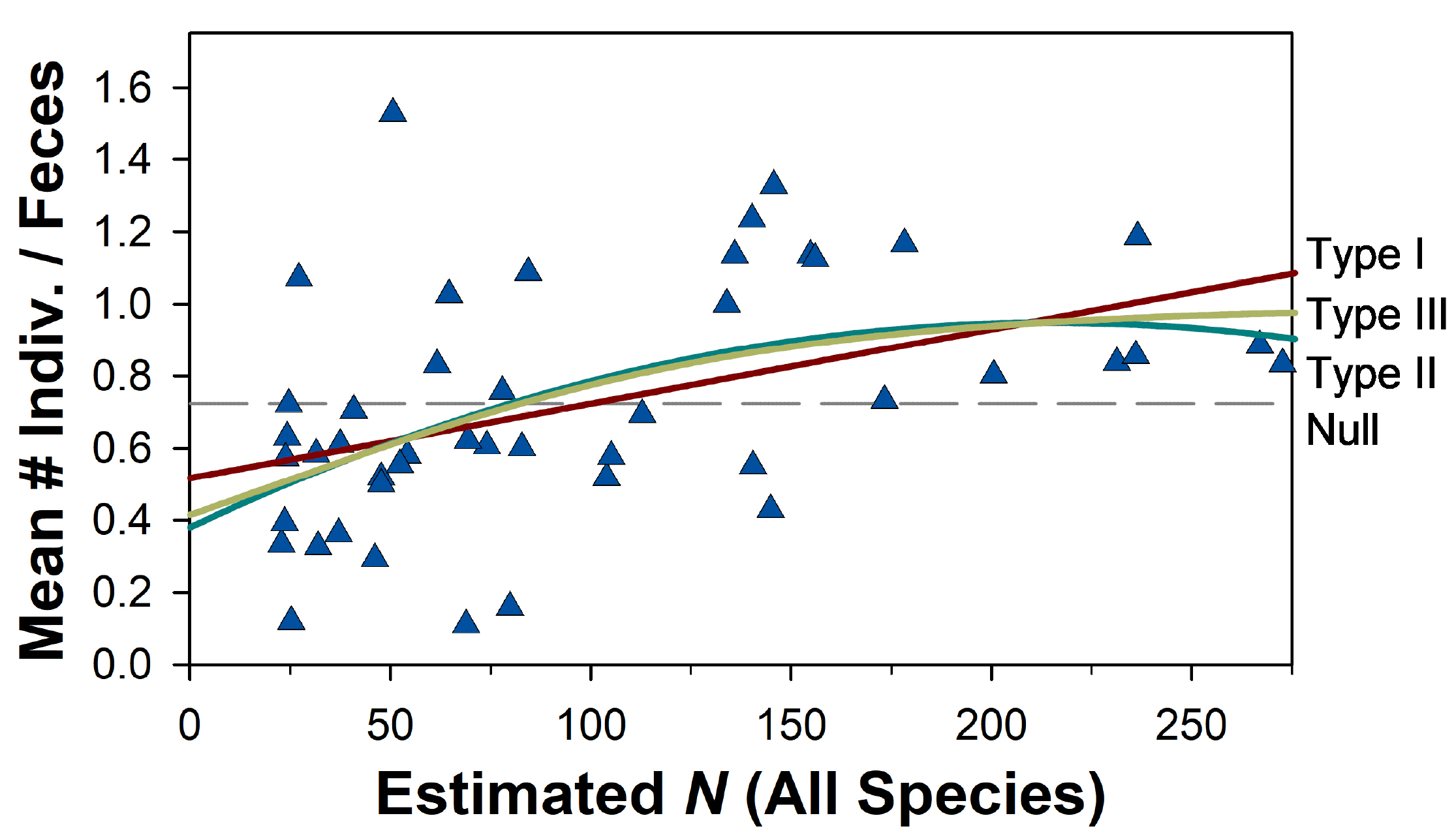
Figure 5. Per capita consumption of small mammals (mean number of individuals per scat) by Culpeo (Lycalopex culpaeus) as a function of estimated population size in 6-month periods over 23 years at Fray Jorge, Chile. Type I, II, and III regressions provide similar representation of these data (∆AICc = 0.34 and 0.64 for the latter two models; see Table 1 for regression results). The principle of parsimony would argue for the simpler model, whereas foraging ecology (e. g., handling time, finite stomach and digestive capacity) suggests that foxes must satiate at some level of prey density; in combination, these arguments suggest that a Type II functional response best expresses Culpeo foraging on small mammals.
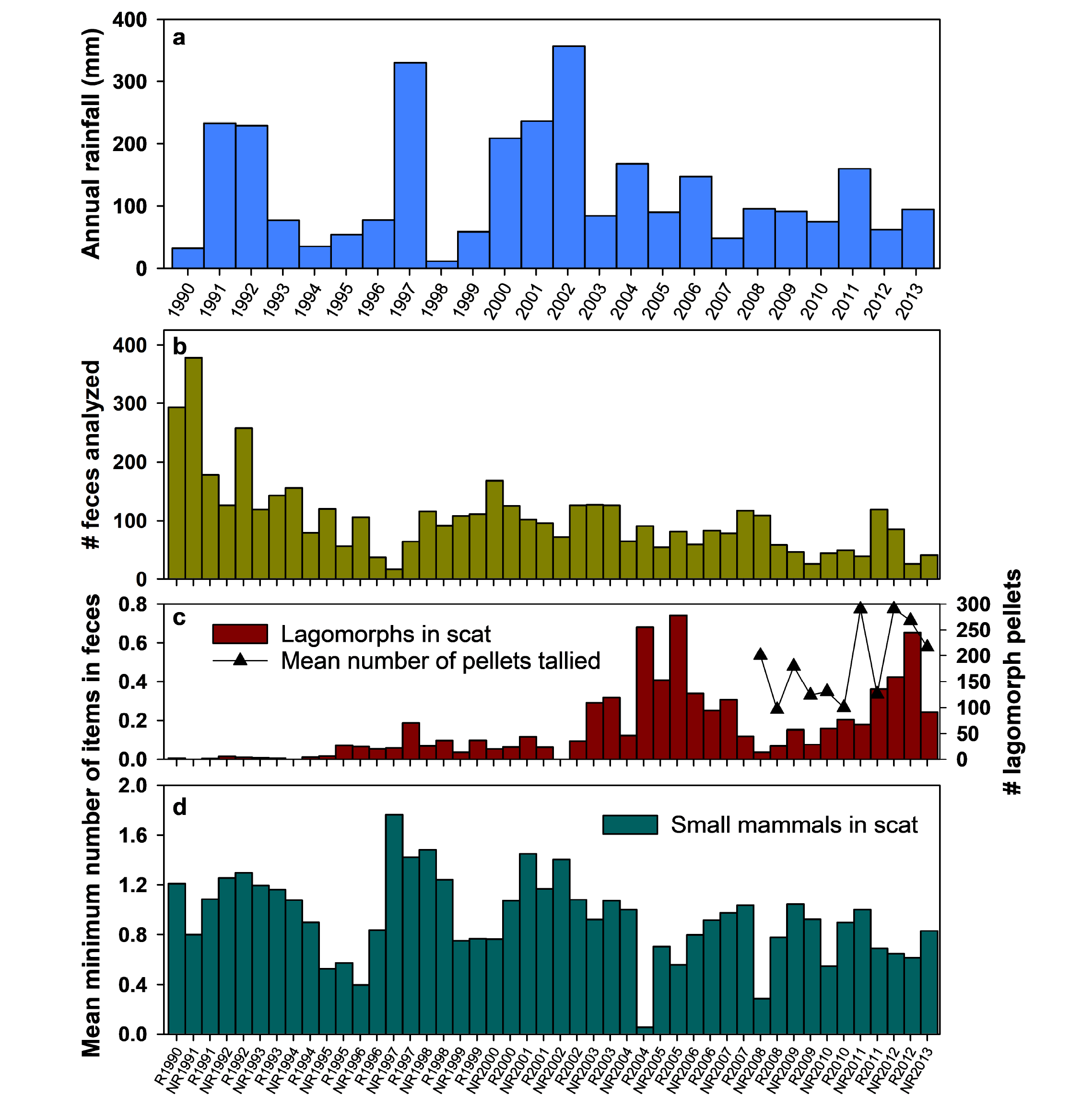
Figure 6. The importance of invasive lagomorphs (Lepus europaeus and Oryctolagus cuniculus) in the diet of Culpeo (Lycalopex culpaeus) became apparent in 2003 (a), following three sequential rainy years and a 2002 outbreak of canid distemper that killed an undetermined portion of the local fox population (Moreira and Stutzin 2005, Acosta Jamett 2010). The number of small mammals (b) fluctuated temporally but did not covary (positively or negatively) with that of lagomorphs. Triangular symbols in panel c present the mean number of lagomorph pellets tallied on standardized stations from 2008 through 2013 (see methods).