THERYA, 2025, Vol. 16(1):41-75 DOI:10.12933/therya-25-6153 ISSN 2007-3364
Genus-level review of pocket gophers in the family Geomyidae
Sergio Ticul Álvarez-Castañeda1*, and Cintya A. Segura-Trujillo2
1 Centro de Investigaciones Biológicas del Noroeste, S.C. Av. Instituto Politécnico Nacional 195, Playa Palo de Santa Rita Sur, CP. 23096, La Paz. Baja California Sur, México. Email: sticul@cibnor.mx (STA-C).
2 Departamento de Bienestar y Desarrollo Sustentable, Centro Universitario del Norte, Universidad de Guadalajara. Carretera Federal No. 23, Km. 191, CP. 46200, Colotlán. Jalisco, México. Email: cintya.segura@academicos.udg.mx (CAS-T).
* Corresponding author: https://orcid.org/0000-0002-2689-8758.
Pocket gophers (Geomyidae) comprise a well-studied family at the species level but need an updated revision at the generic level because studies of each genus have applied different data sets and different criteria for recognizing distinct taxa. Pocket gophers thrive from temperate Canada south to Panama and Colombia, where they inhabit various habitats, including temperate forests, prairies, steppes, hot and cold deserts, and subtropical and tropical areas. The taxonomy at the genus and species levels underwent many changes in the early twenty-first century due to use of different sequencing methodologies. This article builds upon those analyses to review genus-level relationships within the Geomydae. Specifically, we analyzed the sequences available in Genbank for members of the family Geomyidae (Cytb for 47 species and COI for 33 species). We conducted different phylogenetic analyses; in all cases, genera were classified into monophyletic groups associated with the tribes Geomyini and Thomomyini. In the Thomomyini, the genus Thomomys was recognized with two genera, Megascapheus and Thomomys, which are more genetically distinct than many other genera. In the Geomyini, each genus and subgenus are distinct monophyletic groups with very strong support and large p-distances. The Mississippi River appears to function as an important geographic barrier within Geomys, with marked genetic differentiation between populations on the eastern and western sides of the river. Collectively, our analyses based on mtDNA sequences suggest that a more detailed revision employing multiple data sets is needed for the genera within the Geomyidae.
Las tuzas (Geomyidae) comprenden una familia bien estudiada a nivel de especies, pero necesitan una revisión actualizada a nivel de género, debido a que los estudios de cada género han utilizado diferentes conjuntos de datos y criterios para reconocer los distintos taxa. Las tuzas se distribuyen desde Canadá hasta Panamá y Colombia, en diversos hábitats, incluyendo bosques templados, praderas, estepas, desiertos fríos y calientes, así como áreas subtropicales y tropicales. La taxonomía a nivel de género y especie tuvó muchos cambios a principios del siglo veinte y uno debido al uso de diferentes metodologías de secuenciación. Este artículo se basa en esos análisis para revisar las relaciones a nivel de género dentro de los Geomyidae. Específicamente, analizamos las secuencias disponibles en Genbank para especies de la familia Geomyidae (Cytb para 47 especies y COI para 33 especies). Realizamos diferentes análisis filogenéticos; en todos los casos, los géneros fueron clasificados en grupos monofiléticos asociados con las tribus Geomyini y Thomomyini. En los Thomomyini, el género Thomomys fue reconocido con dos géneros, Megascapheus y Thomomys, que son genéticamente más distintos que muchos otros géneros. En los Geomyini, cada género y subgénero son grupos monofiléticos distintos con un fuerte apoyo y grandes distancias p. El río Misisipi parece funcionar como una importante barrera geográfica dentro de Geomys, con una diferenciación genética notable entre las poblaciones en los lados este y oeste del río. Colectivamente, nuestros análisis basados en secuencias de mtDNA sugieren que se necesita una revisión más detallada utilizando múltiples conjuntos de datos para los géneros dentro de los Geomyidae.
Keywords: Geomys; Heterogeomys; Megascapheus; genus; taxonomy; Thomomys.
© 2025 Asociación Mexicana de Mastozoología, www.mastozoologiamexicana.org
Introduction
The family Geomyidae is endemic to the Americas, being distributed from temperate Canada south to Panama and Colombia. Collectively, geomyids occupy a wide range of habitats, from temperate forests, prairies, steppes, hot and cold deserts, and subtropical and tropical areas (Hafner 2017). Over most of their distributions, the different genera of Geomyidae have allopatric distributions (Hall 1981; Hafner 2017). The only areas where two genera are sympatric are in the highlands of central and northern México, where different species of Thomomys and Cratogeomys or Cratogeomys and Zygogeomys co-occur (Russell 1968a; Hall 1981; Patton 2005). The genus- and species-level taxonomy of the Geomyidae underwent multiple changes in the early twenty-first century based on analyses of mitochondrial DNA (mtDNA) and karyotype differences. For example, Cratogeomys and Pappogeomys, both previously considered subgenera of Cratogeomys, were elevated to generic status. Similarly, Heterogeomys and Orthogeomys, both previously considered subgenera of Orthogeomys, were elevated to generic status (Russell 1968a; Hall 1981; Demastes et al. 2002; Spradling et al. 2016).
While the taxonomy and systematics of the Geomyidae have been studied by multiple authors over the past few decades (e. g., Elliot 1903; Russell 1968a; Honeycutt and Williams 1982; DeWalt et al. 1993; Patton 2005; Álvarez-Castañeda 2010; Spradling et al. 2016), many of these studies have focused on a specific genus or group of species within this family. For this reason, there are no consistent criteria that can be used to resolve taxonomic issues for all members of this family. Pocket gophers are a relatively well-studied group at the species level, but a comprehensive revision is needed at the genus level and this may require reconsideration of the criteria used to distinguish between different genera.
Currently, the family Geomyidae is represented by two tribes (Russell 1968a). The tribe Thomomyini includes only the genus Thomomys, with two recognized subgenera, Megascapheus and Thomomys (Elliot 1903). In contrast, the tribe Geomyini (Russell 1968a) includes six genera – Cratogeomys, Geomys, Heterogeomys, Orthogeomys, Pappogeomys, and Zygogeomys. Of these, only Heterogeomys contains subgenera, namely Heterogeomys and Macrogeomys (Russell 1968a). Multiple taxonomic assessments have been completed for each of the currently recognized genera, as exemplified by the following: Cratogeomys (Russell 1968b; DeWalt et al. 1993; Hafner et al. 2004, 2005, 2008), Geomys (Merriam 1895; Hall and Kelson 1959; Russell 1968a; Tucker and Schmidly 1981; Heaney and Timm 1983; Baker et al. 1989; Block and Zimmerman 1991; Jolley et al. 2000; Sudman et al. 2006; Chambers et al. 2009), Heterogeomys (Nelson and Goldman 1929; Russell 1968a; Hall 1981; Patton 2005; Spradling et al. 2016), Orthogeomys (Nelson and Goldman 1929; Russell 1968a; Hall 1981; Patton 2005; Spradling et al. 2016), Pappogeomys (Nelson and Goldman 1934; Russell 1968b; Honeycutt and Williams 1982; Demastes et al. 2002), Thomomys (Hall and Kelson 1959; Anderson 1966, 1972; Patton and Dingmen 1968; Russell 1968a; Thaeler 1968a, b, 1972, 1977, 1980; Hoffmeister 1969, 1986; Patton 1973, 1993, 2005; Thaeler and Hinesley 1979; Patton and Smith 1981, 1990; Hall 1981; Patton et al. 1984; Álvarez-Castañeda 2010; Hafner et al. 2011; Trujano-Álvarez and Álvarez-Castañeda 2013; Mathis et al. 2013a, 2013b, 2014; Álvarez-Castañeda et al. 2017; Bradley et al. 2023), and Zygogeomys (Merriam 1895; Russell 1968a; Hall 1981).
The systematics of the Geomyidae were first established during the late nineteenth to mid- twentieth centuries based on morphology (Merriam 1895; Russell 1968a, b), before the advent of DNA sequencing technologies. Indeed, many of the studies that have contributed to the current taxonomy of the family (see above) pre-date the use of genetic information. Subsequent revisions within each genus or species complex that have employed genetic data have tended to be conducted by multiple groups of researchers employing different criteria to identify genetically distinct taxonomic units. As a result, a comprehensive review of the family that applies consistent sequenced-based criteria to distinguish taxonomic units is lacking. Although mtDNA, nDNA, and karyotypes have all been used to explore geomyid taxonomy (for example, Hafner et al. 2004, 2005, 2008, 2009; Spradling et al. 2016; Sudman et al. 2006; Chambers et al. 2009), the most widely employed genetic marker is the mitochondrial cytochrome b (Cytb) locus. Accordingly, the primary objective of this study was to use Cytb data to evaluate genus-level taxonomic and systematic relationships with the Geomyidae. Delineating generic boundaries requires a well-resolved phylogeny that includes as many species as possible to i) generate a comprehensive overview of current generic names and their type species and ii) clarify generic boundaries and the species that they contain. This article uses Cytb data to create the taxonomic and systematic background required for a rigorous revision generic-level classifications of pocket gophers.
Materials and methods
Sampling and sequencing. Previous studies have generated cytochrome b (Cytb) sequences for nearly all species of geomyids (n = 47 species) as well as cytochrome oxidase subunit 1 (COI) sequences for a somewhat smaller subset of species (n = 33 species); all of these sequences are available in GenBank (Supplementary Material 1). All sequences available for geomids were aligned using the MEGA 11 software package (Tamura et al. 2021). Sequences containing intermediate stop codons were discarded. Analyses were performed using the sequences obtained from both genes. To optimize computational time, we analyzed a subset of five specimens from each monophyletic group, which was average number of samples per species the (Supplementary Material 2); preference was given to sequences that have been used in published taxonomic revisions. Fewer than five sequences were available for some species (Supplemetary Material 2); we were unable to locate sequences for Thomomys idahoensis and T. clusius.
Phylogenetic analyses. Analyses were conducted based on a 1,141-bp fragment of Cytb (n = 169 sequences) and a 1,544-bp fragment of COI (n = 88 sequences; Supplementary Material 1). Because sequence from both Cytb and COI were not available for all species, data from each locus were analyzed separately. Our first analysis assessed the monophyly of each species based on up to five sequences per species. Since sympatry has not been reported for species in the same genus, the source of each sequence was reviewed in detail to avoid confusion between species or potential misidentifications. When multiple sequences were available, preference was given to sequences from localities located farthest from the distribution limits of other species in the same genus; in all cases, efforts were made to select sequences that clearly represented the known geographic distribution of the species in question. Some species are represented by outdated names in GenBank; in some cases, we changed the name of the species following Álvarez-Castañeda (2024) and Bradley et al. (2023; see Supplementary Material 1). Once the monophyly of each species was demonstrated, one sequence per species was selected to construct a representative tree for the family.
Sequence alignments were performed using the MUSCLE software package with default parameters (Edgar 2004). The most suitable evolutionary model for our data set was identified dusing the model comparison software MrModeltest ver. 2 (Nylander 2004) under the Akaike Information Criterion (AIC). Phylogenetic relationships were assessed for each locus using neighbor-joining (NJ), unweighted pair group method with arithmetic mean (UPGMA), maximum-parsimony (MP), maximum-likelihood (ML), and Bayesian inference (BI) optimality criteria. Phylogenetic reconstructions were conducted in PhyML (Guindon et al. 2010), MEGA version 11 (Tamura et al. 2021), and PAUP* version 4.0b (Swofford and Sullivan 2003). A bootstrap consensus tree was inferred from replicates based on uniform rates of the General Time Reversible (GTR) substitution model. Values for percent sequence divergence within and between species were estimated using the uncorrected p-distance parameter model in PAUP. Nodal support was assessed with bootstrap analyses, including a fast heuristic procedure with 1,000 pseudo-replicates (Felsenstein 1985). A Bayesian inference analysis coupled with Markov Chain Monte Carlo (BMCMC) inference was performed in MrBayes v3.2.2 (Ronquist and Huelsenbeck 2003). We carried out two independent BMCMC analyses, each consisting of four chains. Each Markov chain was started from a random tree and run for 10 million generations using the default flat priors, sampling trees every 1,000 generations. Sequence evolution model parameters were treated as unknown variables with uniform default priors and were estimated as part of the analysis. The first 40 % of generations were conservatively deleted as burn-in. Sequences for Chaetodipus californicus, Dipodomys agilis, Heteromys nelsoni, Liomys pictus, Microdipodops pallidus, and Perognathus flavescens were used as outgroups. The outgroup specimens were selected partly following the study of Alexander and Riddle (2005); Genbank accession numbers for outgroup sequences are provided in the supplementary material.
Time calibration. Divergence times between taxa were estimated using BEAST2 v2.6.7 (Bouckaert et al. 2019). For each locus, we implemented three separate Markov Chain Monte Carlo (MCMC) chains to generate a gene tree, with each chain running for 10 million generations. Samples were collected every 1,000 generations to assess the posterior distribution. We set specific priors, including the processed Yule speciation model to account for branching rates, a strict molecular clock to enforce constant rates of evolution across lineages, and a random starting tree to avoid biasing the results (Gernhard et al. 2008). No deep data for the family Geomyidae were found as calibration points. However, because Heteromyidae is the sibling family and a Bayesian divergence dating analysis exists using combined 3-gene data (12S, 16S, and COI), we used 15.9–12.5 mya for Dipodomyinae, 22–20 mya for Perognathinae and 15.2 mya for Heteromyinae (Hafner et al. 2007). To achieve phylogenetic analyses as similar as possible to those reported by Hafner et al. (2007), we used the same specimens in our analyses. After running the chains, we used TreeAnnotator version 10.5.0 to summarize the results and construct a consensus tree. Notably, we applied a burn-in period of 1,000 states to remove any initial inconsistencies in chain convergence and to focus on the most stable estimates of the phylogenetic relationships.
Results
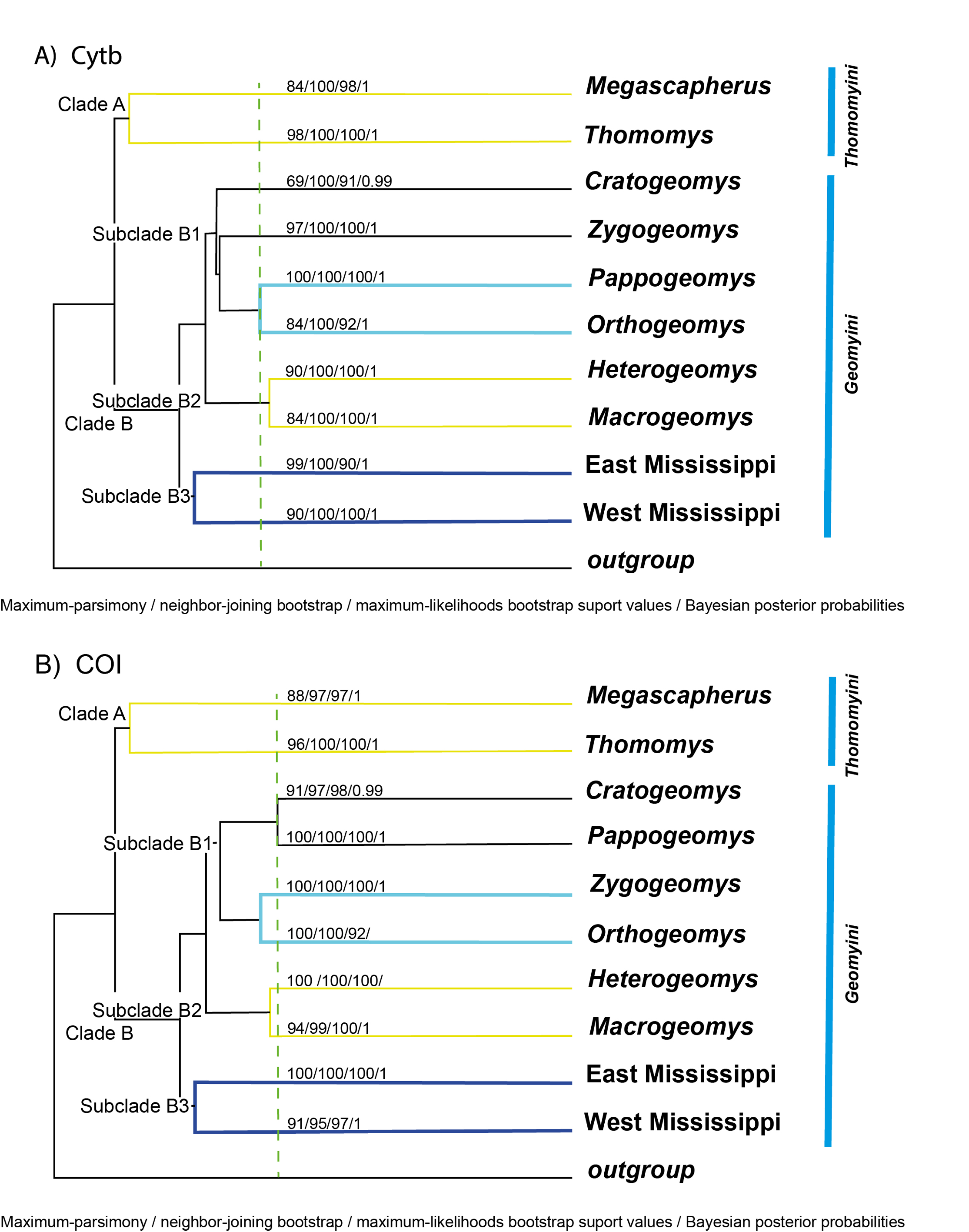
Phylogenetic analyses. The most appropriate evolutionary model for our phylogeny reconstruction was the GTR+I+G model. The model parameters were I = 0.3872 and G = 0.7090, lnL = 28961.9492, k = 10, AIC = 57943.8984. The base frequencies were A = 0.3689, C = 0.3012, G = 0.0502, T = 0.2796, and the relative substitution rates were A–C = 0.4558, A–G = 9.6062, A–T = 0.4878, C–G = 0.4080, C–T = 5.7622, and G–T = 1.0000. All phylogenetic reconstructions for Cytb and COI indicated that Geomyidae is a monophyletic family containing ten monophyletic sub-groups, each of which is markedly divergent from the others and is strongly supported by bootstrap values (Figure 1; Supplementary Material 3). The neighbor-joining (NJ), unweighted pair group method with arithmetic mean (UPGMA), maximum-parsimony (MP), maximum-likelihood (ML), and Bayesian inference (BI; Supplementary Material 3) analyses all produced trees with similar topologies. The same primarly clades were recovered for both the Cytb and COI analyses (Figure 1).
Analyses of both loci revealed that all genera were sorted into two monophyletic groups, each associated with a recognized tribe within the family, namely the Thomomyini (Clade A) and the Geomyini (Clade B), as proposed by Russell (1968a). The tribe Thomomyini includes the single genus Thomomys, which consists of two previously recognized subgenera: Megascapheus and Thomomys. Each subgenus, in turn, contains multiple reciprocally monophyletic groups that are differentiated at the same level and characterized by a high degree of dissimilarity (Tables 1 and 2). Many groups have distinctive morphological characteristics that can be used to differentiate them (see appendix 1). The tribe Geomyini (Clade B) includes three subclades. Subclade B1 consists of four monophyletic groups: Cratogeomys, Pappogeomys, Orthogeomys, and Zygogeomys. This was the only subclade to display differences in tree topology between the Cytb and COI sequences. Subclade B2 is monophyletic and contains the subgenera Heterogeomys and Macrogeomys. The final subclade (B3), which includes all species of Geomys, is split into two monophyletic groups that are distributed on the eastern versus western sides of the Mississippi River (Figure 1).
Percentage of uncorrected p-distance. Percent sequence divergences between species for Cytb and COI sequences analyses are shown in Tables 1 and 2, respectively; percent divergences within species are shown in Table 3. The least divergence between species was found in Cratogeomys, with p-distances ranging from 2.35 % between C. tylorhinus and C. fumosus to 5.00% between C. perotensis and C. merriami. When all ten monophyletic sub-groups of geomyids were considered, percent sequence divergence for Cytb ranged from p = 11.09 % to p = 21.64 % (Table 1); for COI sequences, these values were p = 4.48 % and p = 18.62 % (Table 2). In Clade A, the difference between the two subgenera was 19.92 % (Table 1). In Clade B, sub-clade B1 contains four monophyletic units, each consisting of a single recognized genus. Percent sequence divergences within these genera are as follows: Cratogeomys (p = 15.70 % Cytb, p = 13.09 % COI), Pappogeomys (p = 13.03 % Cytb, p = 13.99 % COI), Orthogeomys (p = 13.03 % Cytb, p = 14.46 % COI), and Zygogeomys (p = 15.38 % Cytb, p = 14.46 % COI). Subclades B2 and B3 contain only two groups each, with p = 11.09 % Cytb and p = 10.44 % COI for Subclade B2 and p = 16.76 % Cytb and p = 14.40 % COI for Subclade B3.
The Geomyidae underwent adaptive radiation during the Cenozoic, resulting in all of the current genera (Álvarez-Castañeda 2024). Our estimates of mitochondrial sequence divergence are consistent with this time frame (Figure 2, 3). Overall, our estimates revealed that the divergence between Heteromyidae and Geomyidae taxa occurred ~ 25.0 mya, placing the crown age for these taxa in the Early Miocene. Within the Geomyidae, estimated divergence times are ~ 14.84 mya for the tribe Thomomyini (Clade A) and ~ 10.82 mya for the Geomyini (Clade B), both of which fall between the Hemingfordian and Barstovian stages of the North American Land Mammal Ages (NALMA) scheme (Wood et al. 1941). Generic-level divergence times range from the Middle Miocene (~ 14 mya) to the Early-Pliocene (~ 4 mya), while most species-level divergence times fall within the Late Pliocene and Pleistocene (3.0 – 0.8 mya). Within Thomomys, divergence of the two subgenera occurred at ~ 7.29 mya for Megascapheus and ~ 7.23 mya for Thomomys. Diversification within the Geomyini began during the Late Miocene (~ 9.0 mya) and continued until the early Pliocene (~ 3.7 mya; Figure 2, 3).
Discussion
The use of different date sets – notably the use of different genetic markers – to examine the taxonomy and systematics of the Geomyidae has made it challenging to develop a comprehensive understanding of diversification within this family. For example, reviews of the different genera of geomyids have tended to employ different combinations of mitochondrial and nuclear genetic markers (Demastes et al. 2002, 2003; Hafner et al. 2009; Mathis et al. 2013a, 2013b; Spradling et al. 2016; Bradley et al. 2023), with the result that data cannot easily be compared across studies. The marker that has been most commonly used across analyses is the mitochondrial Cytb locus and for this reason we have focused our analyses on this gene. Although Cytb tends to reveal relatively low levels of differentiation (i. e., small p-distances) between species, many of these distinctions are supported by data from other genes that serve to validate the separation of species. Here, monophyly was used to establish the degree of differentiation between distinct phylogenetic units; the resulting values for differentiation at the Cytb locus were then used to set boundaries between genera and subgenera, to provide a quantitative basis for distinguishing between taxonomic units at these levels. When possible, these criteria were supplemented by other data sets with potential diagnostic value, including a) time since divergence (estimated ages of clades), b) strength of support (e. g., bootstrap values) for different units, c) genetic distances among other, established taxonomic units within the Geomyidae, and e) morphological differences reported in the literature. Based on these analyses, we suggest that a formal revision of generic-level differentiation within this family is warranted.
Evidence for monophyly. Our phylogenetic analyses provided clear evidence of the monophyly of the ten terminal taxa depicted in Figure 1. With the exception of the distinct eastern and western clades of Geomys depicted in this figure, all other groups have been recognized previously at the generic or sub-generic levels (Demastes et al. 2002; Hafner et al. 2004, 2005, 2008; Sudman et al. 2006; Chambers et al. 2009; Mathis et al. 2013a, 2013b, 2014; Spradling et al. 2016; Álvarez-Castañeda et al. 2017; Bradley et al. 2023). In our analyses, both CytB and COI sequence data provided strong support for these monophyletic units, with 100% support being provided by one or more of the following metrics: neighbor-joining (NJ), unweighted pair group method with arithmetic mean (UPGMA), maximum-parsimony (MP), maximum-likelihood (ML), and Bayesian inference (BI; Figure 1). Monophyly within the genus Geomys has also been documented through the use of three combined genetic regions: the nuclear gene Rbp3, ribosomal RNA (12S rRNA), and mitochondrial DNA (Chambers et al. 2009). These findings align with our analysis, which includes all genera in the family and incorporates the Heteromyidae as an external group. Thus, both our data and those from previous studies indicate that the taxonomic units in Figure 1 are monophyletic. In the case of Thomomys, it had previously been reported based on nuclear genes (seven non-coding nuclear sequence loci) that the monophyly of the species within this genus was not wholly resolved since three of the four named species within the subgenus Thomomys were found to be monophyletic (Belfiore et al. 2008). Our analysis of mitochondrial genes supports the findings of the previously mentioned study regarding the two clades, Megascapheus and Thomomys. Belfiore et al. (2008) proposed that these subgenera originated approximately 5 million years ago (Ma). However, our analysis estimates the origin of these clades to be around 7 Ma. Our estimate aligns with the fossil record for Thomomyines, which date to the middle Hemphillian period (NALMA), also approximately 7 Ma, according to Shotwell (1967) and Tedford et al. (2004).
Genetic differentiation between taxa. At the species level, the least genetic differentiation (i. e., smallest p-distance) evident in our data occurred between Cratogeomys tylorhinus and C. fumosus, which were separated by an average p-distance of 2.35%. Despite their limited divergence, these taxa have been recognized as distinct species based on mtDNA and nDNA analyses (Hafner et al. 2004). Within Geomys, Sudman et al. (2006) and Bradley et al. (2023) employed an estimated percent divergence of ~ 6 % to distinguish between species; this same level of divergence has been applied to Cytb data from other genera of Geomyidae that also included analyses of nuclear markers, karyotypes, and morphology (e. g., Pappogeomys: Demastes et al. 2003; Hafner et al. 2009; Orthogeomys and Heterogeomys: Spradling et al. 2016; Thomomys: Álvarez-Castañeda 2010; Trujano-Álvarez and Álvarez-Castañeda 2013; Mathis et al. 2013a). Our analyses are generally consistent with this 6 % criterion, although several exceptions are evident. One is the small p-distance between C. tylorhinus and C. fumosus noted above. At the other extreme, differentiation with Thomomys townsendi (p = 9.62 %) and T. talpoides (p = 14.61 %) is greater than average values reported for all other species. Although use of p-distances alone to distinguish species is somewhat controversial, revisions based primarily on this metric have been conducted for geomyids, with minimal morphological information used to diagnose species of Geomys (Baker et al. 1989; Block and Zimmerman 1991; Jolley et al. 2000; Sudman et al. 2006; Chambers et al. 2009; Bradley et al. 2023) and Thomomys (Álvarez-Castañeda 2010; Trujano-Álvarez and Álvarez-Castañeda 2013; Álvarez-Castañeda et al. 2017; Bradley et al. 2023). While Patton 2005Beauchamp-Martin et al. (2019) do not recognize Thomomys fulvus as a valid species based on p-distance values, Bradley et al. (2023) not only accept this species but use similar criteria to recognize three other species of Thomomys (T. baileyi, T. connectens, and T. ruidosae). Regardless of whether p-distances alone are considered sufficient for distinguishing species, the range of values for this metric between currently recognized species of geomyids suggests that a species-level revision of these animals is warranted.
At the generic level, our analyses of Cytb sequences indicate that Pappogeomys and Orthogeomys are sibling taxa. These genera, which have long been recognized as distinct based on morphological characteristics (Hall and Kelson 1959; Russell 1968a; Hall 1981; Álvarez-Castañeda 2024), have Cytb a p-distance of 13.03 %, suggesting that this degree of differentiation may provide a basis for defining distinct genera. Based on COI sequences, the most closely related genera are Pappogeomys and Cratogeomys, which are separated by a p-distance of 14 %, providing a potential baseline divergence value for this gene. Although morphological analyses were not conducted as part of this study, information obtained from the literature suggests that with the exception of the east-west split within Geomys, all monophyletic groups reported here (Figure 1) have been recognized previously at the generic or subgeneric levels and that each is associated with a diagnostic description that can be clearly used for identification purposes (Merriam 1895; Nelson and Goldman 1929, 1934;Hall and Kelson 1959; Anderson 1966, 1972; Russell 1968a, b; Hall 1981; Álvarez-Castañeda 2024). Thus, available information indicates that the taxonomic units revealed by analyses of Cytb and COI are robust and should be recognized as distinct.
Review of geomyid taxonomy at the generic level. Our phylogenetic analyses revealed a clear separation between the two standardly recognized sub-clades of geomyids, the tribes Geomyini and Thomomyini (Russell 1968a; Appendix 1).
Tribe Thomomyini. Our analyses indicate that the tribe Thomomyini (our Clade A) is composed of two reciprocally monophyletic clades, each related to one of the subgenera of Thomomys proposed by Thaeler (1980), namely Megascapheus and Thomomys (Patton and Smith 1981, 1989). Megascapheus was proposed by Elliot (1903) but was not recognized by Russell (1968a) or Hall (1981). Our data reveal a clear genetic separation between the proposed sub-genera (p-distance =19.2% for Cytb and 15.46% for COI). Morphologically, Megascapheus species can be distinguished from Thomomys based on a variety of cranial traits (Nelson and Goldman 1934; Russell 1968a; Álvarez-Castañeda 2024); cytogenetically, the number of chromosomes also differs markedly. Thus, overall, our analyses support recognition of Megascapherus and Thomomys as distinct taxonomic units. The degree of genetic differentiation detected between these taxa suggests that they likely should be recognized as distinct genera. concern the skull and teeth, structures normally used as diagnostic attributes at the genus level.
Tribe Geomyini. The Geomyini (our Clade B) was proposed by Russell (1968a) and includes six genera: Cratogeomys, Geomys, Heterogeomys (subgenus Heterogeomys and Macrogeomys), Orthogeomys, Pappogeomys, and Zygogeomys. This scheme has been accepted since it was first proposed, although there have been subsequent modification at the generic and sub-generic levels (Russell 1968a, b; Hall 1981; Spradling et al. 2016). Our analyses indicate that the Genomyini are divided into three reciprocally monophyletic sub-clades. The first of these (our subclade B1) contains the genera Cratogeomys, Pappogeomys, Orthogeomys, and Zygogeomys. Cratogeomys has been considered as a subgenus of Pappogeomys (Russell 1968a, b) but our analyses support those of Demastes et al. (2003) in suggesting that these are distinct genera. Our Cytb analyses suggest that Pappogeomys is sibling to Orthogeomys; this is in contrast to previous work that placed Orthogeomys closer to Zygogeomys (Russell 1968a). Results from other studies indicate that Pappogeomys and Orthogeomys are clearly morphologically different (Merriam 1895; Russell 1968a; Honeycutt and Williams 1982; DeWalt et al. 1993; Demastes et al. 2002, 2003; Spradling et al. 2016; Nelson and Goldman 1934; Álvarez-Castañeda 2024) and thus p-distances between these taxa (p = 13 % for Cytb and 14 % for COI) may provide useful metrics for evaluation the degree of genetic differentiation between other putative genera of geomyids. The final genus in this sub-clade, Zygogeomys, contains only one extant species that is endemic to Michoacán, Mexico; these animals are clearly morphologicall distinct from other members of the sub-clade (Merriam 1895, Russell 1968a, Álvarez-Castañeda 2024), thereby supporting recognition of Zygogeomys as a separate genus.
The second sub-clade of Geomyini (our sub-clade B2) includes the genus Heterogeomys, which consists of two recognized subgenera, Heterogeomys and Macrogeomys (Spradling et al. 2016). Heterogeomys was previously considered a sub-genus of Orthogeomys (Russell 1968a; Hall 1981; Patton 2005), but our analyses indicate that the former is clearly distinct from Orthogeomys. Although relationships among the species in Heterogeomys and Macrogeomys have been reviewed previously (Hafner 1991; Sudman and Hafner 1992), it appears that no detailed analysis has been conducted at the level of these subgenera. Differentiation of Heterogeomys and Macrogeomys is strongly supported by our analyses (Figure 1). Although p-distances between these taxa (11.09 % for Cytb; 10.44 % for COI) are somewhat lower than those reported for Pappogeomys and Orthogeomys, the strong support for the monophyly of these units coupled with documented morphological differences between them (Russell 1968a; Hall 1981) lead us to support their recognition as distinct sub-genera.
The final sub-clade (our sub-clade B3) includes all species of Geomys sensu lato. While our analyses support the monophyly of this genus, they also reveal the presence of two distinct, reciprocally monophyletic lineages that correspond to the eastern and western sides of the Mississippi River (Figure 1). The clade occurring to the east of the Mississippi has been recognized previously as part of the G. pinetis species complex (Russell 1968a; Penney and Zimmerman 1976; Sudman et al. 2006). In contrast, the clade to the west of the Mississippi includes the bursarius and breviceps species groups (Davis 1940; Hall 1981; Sudman et al. 2006). The two lineages display marked variation in cranial morphology (Russell 1968a; Penney and Zimmerman 1976; Sudman et al. 2006), lending further support to the apparent differentiation of these animals. These geographically distinct lineages are characterized by genetic distances (p = 16.72 % for Cytb and 14.66 % for COI) that are larger than those reported here for pairs of established genera (e. g., Pappogeomys-Orthogeomys), suggesting that their inclusion within the single genus Geomys should be reconsidered and the more derived, western lineage potentially elevated to a distinct genus.
Geography of diversification within Geomyidae. No subgenera have been recognized in Geomys, but the analyses in the present study reveal a large p-distance between members of this genus from different sides of the Mississippi River. Animals from west of the river include the bursarius and breviceps species groups, while animals from east of the river include species in the pinetis group (Davis 1940; Hall 1981; Sudman et al. 2006). More broadly, patterns of evolutionary diversification differ markedly between the two recognized tribes of geomyids. While the Thomomyini contains two genera, the Geomyini consists of at least seven genera. The Geomyidae are typically considered to be a fast-evolving group, with much of their diversity emerging during the Late Miocene. This coincides with the expansion of open grass-dominated habitats during the Cenozoic (Strömberg 2011; Anderman et al. 2022), which may have facilitated diversification of these herbivorous rodents. Divergence within the Geomyidae is thought to have been driven by multiple factors, including fluctuations in climatic conditions and their impacts on vegetation, notably the relative expansions and contractions of forests versus grasslands (Castañeda-Rico et al. 2024). At the same time, geographic barriers have no doubt played a role in this dynamic, as has been suggested for the role of the Trans-Mexican Volcanic Belt (TMVB) in determining the distributional limits of multiple genera of geomyids and generating a unique habitat for species in the genera Pappogeomys and Zygogeomys. It seems likely that the Mississippi River has also functioned as an important geographic barrier, particularly within Geomys, which is the only currently recognized genus of pocket gophers to cross this river. Accordingly, it is not surprising that there are marked genetic and morphological differences between members of this genus located on the western versus eastern sides of this major riverine barrier.
Concluding thoughts. Based on our findings that members of the genus Geomys form two genetically distinct lineages that are separated by the Mississippi River, we suggest that formal revision of the genus is warranted, with attention to whether differences between these lineages are sufficient to justify recognition of each as a distinct genus. Our analyses are based on sequence data from two mitochondrial locus indicate that levels of genetic differentiation (p-distances) are greater than those for other pairs of genera within the Geomyidae. These differences are also supported by previous studies describing morphological differences between these lineages. We assert that, given these differences, the two lineages should be formally distinguished using taxonomic categories recognized by the ICZN (1999). We believe that elevation of the western lineage to genus status is more appropriate than description of the two lineages as species groups or complexes as the latter designations lack ICZN (1999) oversight and often add unnecessary complexity to efforts to resolve mammalian taxonomy (Tate 1933; Voss et al. 2014). At the same time, we recognize that an integrated approach –one that makes use of multiple data sets– is critical when diagnosing new taxonomic units. A formal revision of genera within the Geomyidae, in particular evaluation of our proposal that Geomys be divided into two genera, will benefit from examination of nuclear sequence data as well as empirical evaluation of apparent morphological differences between lineages. More generally, significant revision is needed for other portions of the Geomyidae. Although our analyses have focused on generic level differences within this family, revision at other levels is also needed, such as a revision of species-level differentiation with Thomomys. We hope the analyses included here will provide the foundation for a more extensive and comprehensive revision of the taxonomy of the family Geomyidae. Given the ecological and evolutionary importance of these animals, a thorough understanding of their taxonomic diversity should generate critical insights into numerous aspects of mammalian biology.
Acknowledgments
This paper is dedicated to James L. Patton. We gratefully acknowledge the elaboration of the maps by Leticia Cab Sulub. We also thank Noe Gonzalez Ruiz, Eileen Lacey, and four anonymous reviewers for his/her valuable reviews of the manuscript. María Elena Sánchez-Salazar edited the English manuscript.
Literature cited
Andermann, T., et al. 2022. The origin and evolution of open habitats in North America inferred by Bayesian deep learning models. Nature Communications 13:1027 4833.
Alexander, L. F., and B. R. Riddle. 2005. Phylogenetics of the New World rodent family Heteromyidae. Journal of Mammalogy, 86:366-379.
Álvarez, T. 1963. The Recent mammals of Tamaulipas. University of Kansas Publications, Museum of Natural History 14:363–473.
Álvarez-Castañeda, S. T. 2024. Mammals of North America-Systematics and Taxonomy. Volumen 2. Springer.
Álvarez-Castañeda, S. T., and T. Álvarez. 1996. Etimologías de los géneros de mamíferos mexicanos. Ciencia 47:39-49.
Álvarez-Castañeda, S. T. 2010. Phylogenetic structure of the Thomomys bottae-umbrinus complex in North America. Molecular Phylogenetics and Evolution 54:671–679.
Álvarez-Castañeda, S. T., T. Álvarez, and N. González-Ruiz. 2017. Keys for identifying Mexican Mammals. The Johns Hopkins University Press. Baltimore, U.S.A.
Anderson, S. 1966. Taxonomy of gophers, especially Thomomys in Chihuahua, Mexico. Systematic Zoology 15:189–198.
Anderson, S. 1972. Mammals of Chihuahua: taxonomy and distribution. Bulletin of the American Museum of Natural History 148:149–410.
Avise, J. C., et al. 1979. Mitochondrial DNA clones and matriarchal phylogeny within and among geographic populations of the pocket gopher, Geomys pinetis. Proceedings of the National Academy of Sciences 76:6694-6698.
Baker, R. H. 1950. The taxonomic status of Geomys breviceps texensis Merriam and Geomys bursarius llanensis Bailey. Journal of Mammalogy 31:348–349.
Baker, R. H., and B. P. Glass 1951. The taxonomic status of the pocket gophers, Geomys bursarius and Geomys breviceps. Proceedings of the Biological Society of Washington 64:55–58.
Baker, R. J., and S. L. Williams. 1974. Geomys tropicalis. Mammalian Species 35:1-4.
Baker, R. J., and H. H. Genoways. 1975. A new subspecies of Geomys bursarius (Mammalia: Geomyidae) from Texas and New Mexico. Occasional Papers, The Museum, Texas Tech University 29:1–18.
Baker, R. J., et al. 1989. Ribosomal-DNA, mitochondrial-DNA, chromosomal, and allozymic studies on a contact zone in the pocket gopher, Geomys. Evolution 43:63–75.
Baker, R. J., et al. 2003. Pocket gophers (Geomyidae). Pp. 276-286 in Wild Mammals of North America: Biology, Management and Conservation, 2nd ed. The Johns Hopkins University Press. Baltimore, U.S.A.
Belfiore, N. M., L. Liu, and C. Moritz. 2008. Multilocus phylogenetics of a rapid radiation in the genus Thomomys (Rodentia: Geomyidae). Systematic Biology 57:294-310.
Beauchamp-Martin, S. L. et al. 2019. Systematic review of botta’s pocket gopher (Thomomys bottae) from Texas and Southeastern New Mexico, with description of a new taxon. Pp. 515-542, in From field to laboratory: a memorial volume in honor of Robert J. Baker (Bradley, R. D., H. H. Genoways, D. J. Schmidly, and L. C. Bradley, edit.). Special Publications of the Museum of Texas Tech University, Number 71.
Block, S. B., and E. G. Zimmerman. 1991. Allozymic variation and systematics of plains pocket gophers (Geomys) of south-central Texas. The Southwestern Naturalist 36:29–36.
Bradley, R. D., S. K. Davis, and R. J. Baker. 1991. Genetic control of premating-isolating behavior; Kaneshiro’s hypothesis and asymmetrical sexual selection in pocket gophers. Journal of Heredity 82:192–196.
Bradley et al. 2023. Genetic identification of Pocket gophers (Genera Cratogeomys, Geomys, and Thomomys) in Texas and surrounding areas. Special Publications Museum of Texas Tech University 78:1-120.
Bouckaert, R., et al. 2019. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Computational Biology 15:e1006650.
Castañeda-Rico et al. 2024. Unveiling hidden diversity: Phylogenomics of Neotomine rodents and taxonomic implications for the genus Peromyscus. Molecular Phylogenetics and Evolution 203:108233.
Chambers, R. R., P. D. Sudman, and R. D. Bradley. 2009. A phylogenetic assessment of pocket gophers (Geomys): evidence from nuclear and mitochondrial genes. Journal of Mammalogy 90:537-547.
Davis, W. B. 1940. Distribution and variation of pocket gophers (genus Geomys) in the southwestern United States. Texas Agricultural Experiment Station 590:1–38.
Demastes, J. W., et al. 2003. Systematics of a rare species of pocket gopher, Pappogeomys alcorni. Journal of Mammalogy 84:753–761.
Demastes, J. W., et al. 2002. Systematics and phylogeography in pocket gophers in the genera Cratogeomys and Pappogeomys. Molecular Phylogenetics and Evolution 22:144–154.
DeWalt, T. S., et al. 1993. Phylogenetic relationships of pocket gophers (Cratogeomys and Pappogeomys) based on mitochondrial DNA cytochrome b sequences. Molecular Phylogenetics and Evolution 2:193–204.
Elliot, D. G. 1903. A list of mammals obtained by Edmund Heller, collector for the museum, from the coast region of northern California and Oregon. Field Columbian Museum. Zoological series 76: 175-197.
Edgar, R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids research, 32:1792-1797.
Fabre, P. H., et al. 2012. A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evolution Biology 12:88.
Felsenstein, J. 1985. Phylogenies and the comparative method. The American Naturalist 125:1-15.
Gernhard, T., K. Hartmann, and M. Steel. 2008. Stochastic properties of generalised Yule models, with biodiversity applications. Journal of mathematical biology 57:713-735.
Guindon, S., et al. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59:307-321.
Hafner, M. S. 1991. Evolutionary genetics and zoogeography of Middle American pocket gophers, genus Orthogeomys. Journal of Mammalogy 72:1-10.
Hafner, M. 2017. Familia Geomyidae. Pp. 234-269, in Handbook of the Mammals of the World – Volume 6, Lagomorphs and Rodents I (Wilson, D. E., T. E. Lacher, Jr, and R. A. Mittermeier). Lynx Editions, Barcelona, Spain.
Hafner, D. J., et al. 2008. Evolutionary relationships of pocket gophers (Cratogeomys castanops species group) of the Mexican Altiplano. Journal of Mammalogy 89:190–208.
Hafner, M. S., et al. 2004. Systematic revision of pocket gophers of the Cratogeomys gymnurus species group. Journal of Mammalogy 85:1170–1183.
Hafner, M. S., et al. 2005. Cryptic species in the Mexican pocket gopher Cratogeomys merriami. Journal of Mammalogy 86:1095–1108.
Hafner, J. C., et al. 2007. Basal clades and molecular systematics of heteromyid rodents. Journal of Mammalogy 88:1129-1145.
Hafner, M. S., et al. 2009. Evolutionary relationships of pocket gophers of the genus Pappogeomys (Rodentia: Geomyidae). Journal of Mammalogy 90:47–56.
Hafner, M. S., et al. 1994. Disparate rates of molecular evolution in cospeciating hosts and parasites. Science 265 (5175), 1087-1090
Hafner, M. S., et al. 2011. Redescription of the pocket gopher Thomomys atrovarius from the Pacific coast of mainland Mexico. Journal of Mammalogy 92:1367–1382.
Hall, E. R. 1932. Three new pocket gophers from New Mexico and Arizona. Proceedings of the Biological Society of Washington 45: 95–98.
Hall, E. R. 1981. The mammals of North America. Second edition. John Wiley and Sons. New York, U.S.A.
Hall, E. R., and K. R. Kelson. 1959. The mammals of North America. The Ronald Press Co. New York, U.S A.
Heaney, L. R., and R. M. Timm. 1983. Relationships of pocket gophers of the genus Geomys from the central and northern Great Plains. Occasional Papers, Museum of Natural History, University of Kansas 74:1–59.
Hoffmeister, D. F. 1969. The species problem in the Thomomys bottae-Thomomys umbrinus complex of gophers in Arizona. Miscellaneous Publications, Museum of Natural History, University of Kansas 51:75–91.
Hoffmeister, D. F. 1986. Mammals of Arizona. The University of Arizona Press and the Arizona Game and Fish Department. Tucson, U.S.A.
Honeycutt, R. L., and S. L. Williams, 1982. Genic differentiation in pocket gophers of the genus Pappogeomys, with comments on intergeneric relationships in the subfamily Geominae. Journal of Mammalogy 63:208–217.
ICZN. 1999. International code of zoological nomenclature, 4th ed. International Commission for Zoological Nomenclature. London.
Jaeger. E. C. 1955. A source-book of biological names and terms. Third ed. Charles C Thomas, Publisher, Springfield. Illinois, U.S.A.
Jolley, T. W., R. L. Honeycutt, and R. D. Bradley. 2000. Phylogenetic relationships of pocket gophers (genus Geomys) based on the mitochondrial 12s rRNA gene. Journal of Mammalogy 81:1025–1034.
Kennedy, K. 1988. Cospeciation in the host-parasite complex of Geomys pinetis, the southeastern pocket gopher, and chewing lice of the genus Geomydoecus. Doctoral dissertation, Louisiana State University, Baton Rouge.
Komarek, E. V., and D. A. Spencer. 1931. A new pocket gopher from Illinois and Indiana. Journal of Mammalogy 12:404–408.
Lee, H. K., and R. J. Baker. 1987. Cladistical analysis of chromosomal evolution in pocket gophers of the Cratogeomys castanops complex (Rodentia: Geomyidae). Occasional Papers, The Museum, Texas Tech University 114:1-15.
Laerm, J. 1981. Systematic status of the Cumberland Island pocket gopher, Geomys cumberlandius. Brimleyana 6:141-151.
Mclaughlin, C. A. 1958. A new race of the pocket gopher Geomys bursarius from Missouri. Contributions in Science, Los Angeles County Museum 19:1–4.
Mathis, V. L., M. S. Hafner, and D. J. Hafner. 2014. Evolution and phylogeography of the Thomomys umbrinus species complex (Rodentia: Geomyidae). Journal of Mammalogy 95:754–771.
Mathis, V. L., et al. 2013a. Resurrection and redescription of the pocket gopher Thomomys sheldoni from the Sierra Madre Occidental of Mexico. Journal of Mammalogy 94:544–560.
Mathis, V. L., et al. 2013b. Thomomys nayarensis, a new species of pocket gopher from the Sierra del Nayar, Nayarit, Mexico. Journal of Mammalogy 94:983–994.
Merriam, C. H. 1895. Monographic revision of pocket gophers, family Geomyidae (exclusive of the species of Thomomys). North American Fauna 8:1–258.
Nelson, E. W., and E. A. Goldman. 1929. Four new pocket gophers of the genus Heterogeomys from Mexico. Proceedings of the Biological Society of Washington 42:147–152.
Nelson, E. W., and E. A. Goldman. 1934. Revision of the pocket gophers of the genus Cratogeomys. Proceedings of the Biological Society of Washington 47:135–154.
Nylander, J. A. A. 2004. Modeltest v2. Program distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University.
Patton, J. L. 1973. An analysis of natural hybridization between the pocket gophers Thomomys bottae and Thomomys umbrinus, in Arizona. Journal of Mammalogy 54:561–584.
Patton, J. L. 1993. Family Geomyidae. Pp. 469–476, in Mammal species of the world, a taxonomic and geographic reference (Wilson, D. E., and D. M. Reeder, eds.). Second edition. Smithsonian Institution Press, Washington, U.S.A.
Patton, J. L. 2005. Family Geomyidae. Pp. 859–870, in Mammal species of the world. A taxonomic and geographic reference (Wilson, D. E., and D. A. Reeder, eds.). Third edition. The Johns Hopkins University Press. Baltimore, U.S.A.
Patton, J. L., and M. F. Smith. 1981. Molecular evolution in Thomomys: Phyletic systematics, paraphyly, and rates of evolution. Journal of Mammalogy 62:493–500.
Patton, J. L., and M. F. Smith. 1989. Population structure and
the genetic and morphologic divergence among pocket gopher species (Genus Thomomys). Pp. 284-304 in Speciation and its consequences (Otte, D., and J. Endler, eds.) Sinauer, Sunderland. assachusetts, U.S.A.
Patton, J. L., and M. F. Smith. 1990. The evolutionary dynamics of the Pocket Gopher Thomomys bottae, with emphasis on California populations. University of California Publications, zoology 123:1-235.
Patton, J. L., and R. E. Dingman. 1968. Chromosome studies of pocket gophers, genus Thomomys. I. The specific status of Thomomys umbrinus (Richardson) in Arizona. Journal of Mammalogy 49:1–13.
Patton, J. L., et al. 1984. Genetics of hybridization between the pocket gophers Thomomys bottae and Thomomys townsendii in northeastern California. The Great Basin Naturalist 44:431–440.
Penney, D. F., and E. G. Zimmerman 1976. Genic divergence and local population differentiation by random drift in the pocket gopher. Evolution 30:473-483.
Ronquist, F., and J. P. Huelsenbeck. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572-1574.
Russell, R. J. 1968a. Evolution and classification of the pocket gophers of the subfamily Geomyinae. University of Kansas Publications, Museum of Natural History 16:473–579.
Russell, R. J. 1968b. Revision of the pocket gophers of the genus Pappogeomys. University of Kansas Publications, Museum of Natural History 16:581–776.
Shotwell, J. A. 1967. Late Tertiary Geomyoid rodents of Oregon. Bulletin of the Museum of Natural History, University of Oregon. No. 9. Available in: https://hdl.handle.net/1794/20003.
Spradling, T. A., et al. 2016. Systematic revision of the pocket gopher genus Orthogeomys. Journal of Mammalogy 97:405–423.
Spradling, T. A., et al. 2004. DNA data support a rapid radiation of pocket gopher genera J. Mammal. Evol. 11, 105-125.
Strömberg, C. A. E. 2011. Evolution of grasses and grassland ecosystems. Annual Review Earth Planet Sciences 39:517–544.
Sudman, P. D., J. R. Choate, and E. G. Zimmerman. 1987. Taxonomy of chromosomal races Geomys bursarius lutescens Merriam. Journal of Mammalogy 68:526–543.
Sudman, P. D., and, M. S. Hafner. 1992. Phylogenetic relationships among Middle American pocket gophers (genus Orthogeomys) based on mitochondrial DNA sequences. Molecular phylogenetics and evolution 1:17-25.
Sudman, P. D., et al. 2006. Molecular systematics of pocket gophers of the genus Geomys. Journal of Mammalogy 87:668–676.
Swofford, D. L., and J. Sullivan. 2003. Phylogeny inference based on parsimony and other methods using PAUP*. The phylogenetic handbook: a practical approach to DNA and protein phylogeny 7:160-206.
Tamura, K., G. Stecher, and S. Kumar. 2021. MEGA11: molecular evolutionary genetics analysis version 11. Molecular biology and evolution 38:3022-3027.
Tate, G. H. H. 1933. A systematic revision of the marsupial genus Marmosa with a discussion of the adaptive radiation of the murine opossums (Marmosa). Bulletin of the American Museum of Natural History 66:1–250.
Tedford, R. H., et al. 2004. Mammalian biochronology of the Arikareean through Hemphillian interval (late Oligocene through early Pliocene epochs). Pp. 169–231. in Late Cretaceous and Cenozoic mammals of North America: Biostratigraphy and geochronology (Woodburne, M. O. ed.). Columbia University Press. New York, U.S.A.
Thaeler, C. S., Jr. 1968a. An analysis of three hybrid populations of pocket gophers (genus Thomomys). Evolution 22:543–555.
Thaeler, C. S., Jr. 1968b. An analysis of the distribution of pocket gopher species in northeastern California (genus Thomomys). University of California Publlications, Zoology 86:1-46.
Thaeler, C. S., Jr. 1972. Taxonomic Status of the Pocket Gophers, Thomomys idahoensis and Thomomys pygmaeus (Rodentia, Geomyidae). Journal of Mammalogy 53:417–428.
Thaeler, C. S., Jr. 1977. Taxonomic status of Thomomys talpoides confinus. Murrelet 58:49–50.
Thaeler, C. S., Jr. 1980. Chromosome numbers and systematics relations in the genus Thomomys (Rodentia: Geomyidae). Journal of Mammalogy 61:414–422.
Thaeler, C. S., Jr., and L. L. Hinesley. 1979. Thomomys clusius, a rediscovered species of pocket gopher. Journal of Mammalogy 60:480–488.
Trujano-Álvarez, A. L., and S. T. Álvarez-Castañeda. 2013. Phylogenetic structure among pocket gopher populations, genus Thomomys (Rodentia: Geomyidae), on the Baja California Peninsula. Zoological Journal of the Linean Society 168:873–891.
Tucker, P. K., and D. J. Schmidly. 1981. Studies on a contact zone among three chromosomal races of Geomys bursarius in east Texas. Journal of Mammalogy 62:258–272.
Voss, R. S., et al. 2014. Phylogenetic relationships of mouse opossums (Didelphidae: Marmosa) with a revised subgeneric classification and notes on sympatric diversity. American Museum Novitates 3817:1–27.
Williams, S. L., and H. H. Genoways. 1980. Morphological variation in the southeastern pocket gopher, Geomys pinetis (Mammalia: Rodentia). Annals of Carnegie Museum 49:405–453.
Wood, H. E., et al. 1941. Nomenclature and correlation of the North American continental Tertiary. Geological Society of America Bulletin 52:1-48.
Associated editors: Marjorie Matocq and Eileen Lacey
Submitted: September 14, 2024; Reviewed: December 7, 2024
Accepted: January 20, 2025; Published on line: January 31, 2025
Appendix 1
SYSTEMATICS
We propose that pocket gophers in the family Geomyidae be classified into nine genera, as defined, diagnosed, and discussed in the following generic accounts. For the sake of reference completeness, the literature cited provides the full citations for each of the generic-level names (Merriam 1895; Elliot 1903).
Tribe Thomomyini (Clade A)
Genus Megascapheus Elliot 1903
1903. Megascapheus Elliot, Field Columb. Mus., Publ. 76, Zool. Ser., 3(11):190. Type species. Diplostoma bulbivorum (Richardson, 1829).
Content. Thirteen allopatric species of Megascapheus are recognized: Megascapheus atrovarius (Allen, 1898), Megascapheus baileyi (Merriam, 1901), Megascapheus bottae (Eydoux and Gervais, 1836), Megascapheus bulbivorus (Richardson, 1829), Megascapheus connectens (Hall, 1936), Megascapheus fulvus (Woodhouse, 1852), Megascapheus laticeps (Baird, 1855), Megascapheus nayarensis (Mathis et al., 2013), Megascapheus nigricans (Rhoads, 1895), Megascapheus ruidosae (Hall, 1932), Megascapheus sheldoni (Bailey, 1915), Megascapheus townsendii (Bachman, 1839), and Megascapheus umbrinus (Richardson, 1829).
Etymology. The name Megascapheus is derived from the Greek Mega, meaning “great” and skapherus, “a digger”: the great digger (Jaeger 1955).
Diagnosis. The species in the genus Megascapheus can be distinguished from those in the genus Thomomys by having the upper incisors procumbent and their root between the fourth upper premolars and the first upper molars; sphenoidal fissure open; angular process continuous, with a well-developed flange along the ventral ramus side; rostrum heavy; base of the first lower premolars inclined anteriorly; infraorbital canal openings anterior to the incisive foramina; anterior enamel plate of the first lower premolars not recurved; anterior enamel plate of the first lower premolars narrow and broadly separated from the lateral enamel plate on the lingual side; chromosome number of living forms from 74 to 82 (Nelson and Goldman 1934; Russell 1968a; Álvarez-Castañeda 2024).
Distribution. Megascapheus ranges from southern Oregon, Idaho, and Colorado southward through Michoacán, State of Mexico, Mexico City, Puebla, and Veracruz and eastward through New Mexico, Texas, Coahuila, Nuevo Leon, San Luis Potosí, and Veracruz.
Comments. Megascapheus and Thomomys were considered two subgenera of Thomomys, with clear morphological differences. Morphological and genetic data support considering these differences sufficient at the genus level. The subspecies of M. fulvus should be reviewed, increasing the number of sequences of the recognized subspecies to carry out a detailed analysis. The nomenclature used in the genus is supported in the studies of Hall and Kelson (1959), Anderson (1966, 1972), Patton and Dingmen (1968), Thealer (1968a, b, 1972, 1977, 1980), Hoffmeister (1969, 1986), Patton (1973, 1993, 2005), Thaeler and Hinesley (1979), Patton and Smith (1981, 1990), Patton et al. (1984), Álvarez-Castañeda (2010), Hafner et al. (2011), Trujano-Álvarez and Álvarez-Castañeda (2013), Mathis et al. (2013a, 2013b, 2014), Álvarez-Castañeda et al. (2017), and Bradley et al. (2023).
Morphological difference between Megascapheus in relation to Thomomys are: the upper incisors procumbent and their root between the fourth upper premolars and the first upper molars vs upper incisors not procumbent and their root above the fourth upper premolars; sphenoidal fissure open vs closed (except for some specimens of T. clusius); angular process continuous, with a well-developed flange along the ventral ramus side vs not continuous, with a weakly developed flange along the ventral ramus side; rostrum heavy vs rostrum slender; base of the first lower premolars inclined anteriorly vs nearly perpendicular to the occlusal surface of the toothrow; infraorbital canal openings anterior to the incisive foramina vs directly above or slightly posterior to the incisive foramina; anterior enamel plate of the first lower premolars not recurved vs recurved, frequently forming a shallow re-entrant angle; anterior enamel plate of the first lower premolars narrow and broadly separated from the lateral enamel plate on the lingual side, vs only slightly separated from the posterior enamel plate (rarely continuous), with the lateral enamel plate on the lingual side; chromosome number of living forms from 74 to 82 vs from 40 to 60 (Nelson and Goldman 1934; Russell 1968a; Álvarez-Castañeda 2024).
Megascapheus atrovarius (Allen, 1898)
(Southern pocket gopher, tuza de Sinaloa)
1898. Thomomys atrovarius (J. A. Allen), Bull. Amer. Mus. Nat. Hist., 10:148. Type locality “Tatemales (near Rosario), Sinaloa”.
2024. Megascapheus atrovarius: (this study).
1. M. a. atrovarius (Allen, 1898). For type locality see above. Range from the central Sinaloa coast south through northwestern Jalisco.
2. M. a. parviceps (Nelson and Goldman, 1934). Type locality “Chacala, 3000 ft., Durango”. Known only from central and northeastern Sinaloa and western Durango.
3. M. a. simulus (Nelson and Goldman, 1934). Type locality “Alamos, 1200 ft., Sonora”. Known only from southeastern Sonora and northeastern Sinaloa.
4. M. a. sinaloae (Merriam, 1901). Type locality “Altata, Sinaloa”. Range in coastal central and northern Sinaloa.
Megascapheus baileyi (Merriam, 1901)
Southwestern Texas pocket gopher, tuza del suroeste de Texas
1901. Thomomys baileyi Merriam, Proc. Biol. Soc. Washington, 14:109. Type locality “Sierra Blanca, Hudspeth Co., Texas”.
2024. Megascapheus baileyi: (this study).
1. M. b. actuosus (Kelson, 1951). Type locality " Corona, Lincoln Co., New Mexico”. Known only from central New Mexico.
2. M. b. analogus (Goldman, 1938a). Type locality " Sierra Guadalupe, about 12 mi. S General Cepeda, Coahuila”. Range in southeastern Coahuila, southwestern Coahuila, and Nuevo León.
3. M. b. baileyi (Merriam, 1901). For type locality see above. Known only from type locality.
4. M. b. confinalis (Goldman, 1936). Type locality " 35 mi. E Rock Springs, 2450 ft., Texas”. Known only from Sutton County, southwestern Texas.
5. M. b. cultellus (Kelson, 1951). Type locality " Halls Peak, Mora Co., New Mexico”. Known only from Mora County, northeastern New Mexico.
6. M. b. guadalupensis (Goldman, 1936). Type from McKittrick Canyon, 7800 ft., Guadalupe Mts., Texas.” Known only from Guadalupe Mountains New Mexico and Texas.
7. M. b. humilis (Baker, 1953). Type locality " 3 mi. W Hda. San Miguel, 2200 ft., Coahuila”. Known only from northern Coahuila.
8. M. b. lachuguilla (Bailey, 1902). Type locality " arid foothills near El Paso, El Paso Co., Texas”. Range in southern New Mexico and western Texas.
9. M. b. limitaris (Goldman, 1936). Type locality “4 mi. W Boquillas, Brewster Co., Texas”. Known only from southwestern Texas.
10. M. b. limpiae (Blair, 1939). Type locality " Limpia Canyon, 1 mi. N Fort Davis, 4700 ft., Jeff Davis Co., Texas”. Known only from Jeff Davis County, western Texas.
11. M. b. opulentus (Goldman, 1935). Type locality " Las Palomas, on the Rio Grande, Sierra Co., New Mexico”. Known only from Sierra County, central New Mexico.
12. M. b. pectoralis (Goldman, 1936). Type locality “Vicinity of Carlsbad Cave, Carlsbad Cave National Monument, Eddy Co., New Mexico”. Known only from Carlsbad Cave National Monument.
13. M. b. pervagus (Merriam, 1901). Type locality “Española, Rio Arriba Co., New Mexico”. Known from northern New Mexico.
14. M. b. retractus (Baker, 1953). Type locality “Fortin, 3300 ft., 20 mi. N, 2 mi. E San GerOnimo, Coahuila”. Known only from northern Coahuila.
15. M. b. robertbakeri (Beauchamp-Martin et al. 2019). Type locality “2.5 mi. E McCamey, Upton County, Texas”. Known only from southern-central Texas.
16. M. b. scotophilus (Davis, 1940). Type locality “1 1/2 W Bat Cave, Sierra Diablo, Hudspeth Co., Texas. Known only from Sierra Diablo Texas.
17. M. b. spatiosus (Goldman, 1938). Type locality “Alpine, 4500 ft., Brewster Co., Texas”. Known only from Brewster County, western Texas.
18. M. b. sturgisi (Goldman, 1938a). Type locality “Sierra del Carmen, 6000 ft., Coahuila”. Range from central Coahuila to northwestern Coahuila.
19. M. b. texensis (Bailey, 1902). Type locality “head of Limpia Creek, 5500 ft., Davis Mts., Jeff Davis Co., Texas”. Known only from southwestern Texas.
20. M. b. tularosae (Hall, 1932). Type locality “Cook Ranch, ½ mi. W Tularosa, Otero Co., New Mexico”. Known only from Tularosa are in New Mexico.
21. M. b. villai (Baker, 1953). Type locality “7 mi. S, 2 mi. E Boquillas, 1800 ft., Coahuila”. Known only from type locality.
Megascapheus bottae (Eydoux and Gervais, 1836)
Botta’s pocket gopher, tuza del norte
1836. Oryctomys (Saccophorus) bottae Eydoux and Gervais, Mag. de Zool., Paris, 6:23. Type locality “Coast of California”; name applied by Baird (Proc. Acad. Nat. Sci. Philadelphia, 7:335) to the gopher occurring in vie. Monterey.
1966. Thomomys bottae: Anderson, Syst. Zool., 15:192.
2024. Megascapheus bottae: (this study).
1. M. b. bottae (Eydoux and Gervais, 1836). For type locality see above. Range in western coast of California from San Francisco Bay south through Ventura County.
2. M. b. mewa (Merriam, 1908). Type locality “Raymond, Madera Co., California”. Known only from Maderas County, central California.
3. M. b. navus (Merriam, 1901). Type locality “Red Bluff, Tehama Co., California”. Known only from Tehama County, northern California.
4. M. b. pascalis (Merriam, 1901). Type locality “Fresno, San Joaquin Valley, Fresno Co., California”. Known only from Fresno County, central California.
Megascapheus bulbivorus (Richardson, 1829)
Camas pocket gopher, tuza del Valle de Camas
1829. Diplostoma bulbivorum Richardson, Fauna Boreali Americana, 1:206. Type locality "Banks of the Columbia River, Oregon," probably Portland, the only place near the Columbia River where it has been taken since. The type was reported as in the Hudson Bay Museum but has not been found (fide V. Bailey, N. Amer. Fauna, 39:40, November 15, 1915).
1855. Thomomys bulbivorus: Beiträge zur nähern Kenntniss der Säugethiere Russland's. St. Pétersburg Acad. Sci. Mem., 9:188.
2024. Megascapheus bulbivorus: (this study).
Megascapheus connectens (Hall, 1936)
(New Mexico pocket gopher, tuza de Nuevo Mexico)
1936. Thomomys umbrinus connectens Hall, Jour. Washington Acad. Sci., 26:296. Type locality " Clawson Dairy, 5 mi. N Albuquerque, 4943 ft., Bemalillo Co., New Mexico”. Known only from Bernalillo County, New Mexico.
2024. Megascapheus connectens: (this study).
Megascapheus fulvus (Woodhouse, 1852)
Fulvus pocket gopher, tuza del suroeste
1852. Geomys fulvus Woodhouse, Proc. Acad. Nat. Sci. Philadelphia, 6:201. Type locality “San Francisco Mtn., Coconino Co., Arizona”.
2010. Thomomys fulvus: Álvarez-Castañeda, Mol. Phylog. Evol., 54:679.
2024. Megascapheus fulvus: (this study).
1. M. f. abstrusus (Hall and Davis, 1935). Type locality " Fish Spring Valley, 2 mi. SE Tulle Peak, 7000 ft., Nye Co., Nevada”. Known only from Nye County, southern Nevada.
2. M. f. albicaudatus (Hall, 1930). Type locality " Provo, 4510 ft., Utah Co., Utah”. Known only from Utah County, central Utah.
3. M. f. alexandrae (Goldman, 1933b). Type locality " plain 5 mi. SW Rainbow Lodge, near Navajo Mtn., 6200 ft., Coconino Co., Arizona”. Known only from Coconino County, northern Arizona.
4. M. f. alpinus (Merriam, 1897). Type locality " Big Cottonwood Meadows, 10,000 ft., 8 mi. SE Mt. Whitney peak, High Sierra, Inyo Co., California”. Known only from Mount Whitney, Inyo County, western California.
5. M. f. angustidens (Baker, 1953). Type locality " Sierra del Pino, 5250 ft., 6 mi. N, 6 mi. W Acebuches, Coahuila”. Known only from Sierra del Pino, Coahuila.
6. M. f. apache (Bailey, 1910). Type locality " Lake La Jara, 7500 ft., Jicarilla Apache Indian Reservation, New Mexico”. Known only from Sandoval County, northern New Mexico.
7. M. f. aureiventris (Hall, 1930). Type locality " Fehlman Ranch, 3 mi. N Kelton, 4225 ft., Boxelder Co., Utah”. Known only from Box Elder County, northern Utah.
8. M. f. aureus (Allen, 1893). Type locality " Bluff City, San Juan Co., Utah”. Known only from San Juan County, southwestern Utah.
9. M. f. basilicae (Benson and Tillotson, 1940). Type locality " La Misi6n, 2 mi. W Magdalena, Sonora”. Known only from central Sonora.
10. M. f. birdseyei (Goldman, 1937a). Type locality " Pine Valley Mts., 5 mi. E Pine Valley, 8300 ft, Washington Co., Utah”. Restricted to Washington County, southwestern Utah.
11. M. f. bonnevillei (Durrant, 1946). Type locality " Fish Springs, 4400 ft., Juab Co., Utah”. Known only from Juab County, western Utah.
12. M. f. brevidens (Hall, 1932a). Type locality " Breen Creek, 7000 ft., Kawich Range, Nye Co., Nevada”. Known only from Nye County, southern Nevada.
13. M. f. camoae (Burt, 1937). Type locality " Camoa, Rio Mayo, Sonora”. Known only from central-southern coast Sonora.
14. M. f. canus (Bailey, 1910). Type locality " Deep Hole, N end Smoke Creek Desert, Washoe Co., Nevada”. Known only from Smoke Creek Desert, Washoe County, northwestern Nevada.
15. M. f. catalinae (Goldman, 1931). Type locality " Swnmerhaven, Santa Catalina Mts., 7500 ft., Pima Co., Arizona”. Known only from Pima County, southern Arizona.
16. M. f. cervinus (Allen, 1895). Type locality " Phoenix, Maricopa Co., Arizona”. Known only from Maricopa County, southwestern Arizona.
17. M. f. cinereus (Hall, 1932a). Type locality " West Walker River, Smiths Valley, 4700 ft., Lyon Co., Nevada”. Known only from Lyon County, Nevada.
18. M. f. collis (Hooper, 1940). Type locality " Shuman's Ranch, 30 mi. S Grants, sec. 30, T. 6 N, R. 10 W, Valencia Co., New Mexico”. Known only from Valencia County, western New Mexico.
19. M. f. concisor (Hall and Davis, 1935). Type locality " Pott's Ranch, 6900 ft., Monitor Valley, Nye Co., Nevada”. Known only from Nye County, southern Nevada.
20. M. f. contractus (Durrant, 1946). Type locality " Scipio, 5315 ft., Millard Co., Utah”. Known only from Millard County, western Utah.
21. M. f. convergens (Nelson and Goldman, 1934)”. Type locality " Costa Rica Ranch, delta Sonora River, SW of Hermosillo, Sonora”. Known only from the Sonora River delta, Sonora.
22. M. f. convexus (Durrant, 1939). Type locality " E side Clear Lake, 4600 ft., Millard Co., Utah”. Known only from Millard County, western Utah.
23. M. f. cultellus (Kelson, 1951). Type locality " Halls Peak, Mora Co., New Mexico”. Known only from Mora County, northeastern New Mexico.
24. M. f. curtatus (Hall, 1932a). Type locality " San Antonio, 5400 ft., Nye Co., Nevada”. Known only from Nye County, southern Nevada.
25. M. f. depressus (Hall, 1932a). Type locality " Dixie Meadows (at S end Humboldt Salt Marsh), 3500 ft., Churchill Co., Nevada”. Known only from Churchill County, western Nevada.
26. M. f. desertorum (Merriam, 1901). Type locality " Mud Spring, Detrital Valley, Mohave Co., Arizona”. Known only from Detrital Valley, Mohave Country, southeastern Arizona.
27. M. f. dissimilis (Goldman, 1931). Type locality " E slope Mt. Ellen, 8000 ft., Henry Mts., Garfield Co., Utah”. Known only from Garfield County, southern Utah.
28. M. f. divergens (Nelson and Goldman, 1934). Type locality " 4 mi. W Huachinera, 4000 ft., Rio Bavispe, Sonora”. Known only from Huachinera, eastern Sonora.
29. M. f. estanciae (Benson and Tillotson, 1939). Type locality " La Estancia, 6 mi. N Nacori, Sonora”. Known only from Nácori, eastern Sonora.
30. M. f. fulvus (Woodhouse, 1852). For type locality see above. Range from central Arizona to western New Mexico.
31. M. f. fumosus (Hall, 1932a). Type locality " Milman Ranch, Moores Creek, 19 mi. SE Millett P.O., Nye Co., Nevada”. Known only from Nye County, southern Nevada.
32. M. f. howelli (Goldman, 1936). Type locality " Grand Junction, 4600 ft., Mesa Co., Colorado”. Known only from Mesa County, western Colorado.
33. M. f. internatus (Goldman, 1936). Type locality " Salida, 7000 ft., Chaffee Co., Colorado”. Known only from Chaffee County, southern Colorado.
34. M. f. lacrymalis (Hall, 1932a). Type locality " Arlemont [Chiatovich Ranch, Fish Lake Valley], 4900 ft., Esmeralda Co., Nevada”. Known only from Esmeralda County, western Nevada.
35. M. f. latus (Hall and Davis, 1935). Type locality " Cherry Creek, 6500 ft., White Pine Co., Nevada”. Known only from White Pine County, eastern Nevada.
36. M. f. lenis (Goldman, 1942). Type locality " Richfield, 5308 ft., Sevier Co., Utah”. Known only from Sevier County, central Utah.
37. M. f. levidensis (Goldman, 1942). Type locality " Manti, about 5500 ft., Sanpete Co., Utah”. Known only from Sanpete County, central Utah.
38. M. f. lucrificus (Hall and Durham, 1938)”. Type locality " Eastgate, Churchill Co., Nevada”. Known only from Churchill County, western Nevada.
39. M. f. mearnsi (Bailey, 1914). Type locality " Grays Ranch, 5000 ft., Animas Valley, Grant Co., New Mexico”. Known only from the southwest corner of New Mexico.
40. M. f. minimus (Durrant, 1939). Type locality " Stansbury Island, Great Salt Lake, Tooele Co., Utah”. Known only from Tooele County, northwestern Utah.
41. M. f. modicus (Goldman, 1931). Type locality " La Osa (near Mexican boundary), southern end of Altar Valley, Pima Co, Arizona”. Known only from northern Sonora.
42. M. f. morulus (Hooper, 1940). Type locality " Bill Porter's Ranch, 8 mi. SE Paxton, Valencia Co., New Mexico”. Only known of Cibola County, western New Mexico.
43. M. f. nanus (Hall, 1932a). Type locality " S end Belted Range, 5½ mi. NW White Rock Spring, 7200 ft, Nye Co., Nevada”. Known only from Nye County, southern Nevada.
44. M. f. nesophilus (Durrant, 1936). Type locality " Antelope Island, Great Salt Lake, Davis Co., Utah”. Known only from Antelope Island, Davis County, northern Utah.
45. M. f. operarius (Merriam, 1897). Type locality " Keeler, E side Owens Lake, Inyo Co., California”. Ony knoen from Inyo County, eastern California.
46. M. f. operosus (Hatfield, 1942). Type locality " Peeples Valley, 4400 ft., 6 mi. N Yarnell, Yavapai Co., Arizona”. Known only from Yavapai County, central Arizona.
47. M. f. optabilis (Goldman, 1936). Type locality " Coventry, 6500 ft., Montrose Co., Colorado”. Known only from Montrose County, western Colorado.
48. M. f. osgoodi (Goldman, 1931). Type locality " Hanksville, Wayne Co., Utah”. Known only from Wayne County, central Utah.
49. M. f. paguatae (Hooper, 1940). Type locality " ½ mi. N Cebolleta (Seboyeta P.O.), Valencia Co., New Mexico”. Known only from Valencia County, western central New Mexico.
50. M. f. peramplus (Goldman, 1931). Type locality “Wheatfields Creek, 7000 ft. [about 27 mi. E Chin Lee], W slope Tunitcha Mts., Apache Co., Arizona”. Known only from Apache County, northwestern Arizona.
51. M. f. perditus (Merriam, 1901). Type locality “Lampazos, Nuevo León”. Range from eastern Coahuila and western Nuevo León.
52. M. f. perpallidus (Merriam, 1886). Type locality “Palm Springs, Riverside Co., California”. Known only from Riverside County, southern California.
53. M. f. pervagus (Merriam, 1901). Type locality “Española, Rio Arriba Co., New Mexico”. Known only from Rio Arriba and Santa Fe counties, northern New Mexico.
54. M. f. phelleoecus (Burt, 1933). Type locality “Hidden Forest, 8500 ft., Sheep Mts., Clark Co., Nevada”. Known only from Clark County, southern Nevada.
55. M. f. pinalensis (Goldman, 1938b). Type locality “Oak Flat, 5 mi. E Superior, Pinal Mts., Pinal Co., Arizona”. Known only from Gila County, central Arizona.
56. M. f. planirostris (Burt, 1931). Type locality “Zion National Park, Washington Co., Utah”. Known only from Washington County, southwestern Utah.
57. M. f. planorum (Hooper, 1940). Type locality “1 ½ mi. SW San Mateo, Valencia Co., New Mexico”. Known only from Valencia County, western-central New Mexico.
58. M. f. powelli (Durrant, 1955). Type locality “Hall Ranch, Salt Gulch, 8 mi. W Boulder, 6000 ft., Garfield Co., Utah”. Known only from Garfield County, southern Utah.
59. M. f. pusillus (Goldman, 1931). Type locality “Coyote Mis., 3000 ft., Pima Co., Arizona”. Known only from Pima County, southern Arizona.
60. M. b. riparius (Grinnell and Hill, 1936a). Type locality “Blythe, Riverside Co., California”. Known only from Riverside County, southern California.
61. M. f. robustus (Durrant, 1946). Type locality “Orr's Ranch, 4300 ft., Skull Valley, Tooele Co., Utah”. Known only from Tooele County, northwestern Utah.
62. M. f. sevieri (Durrant, 1946). Type locality “Swasey Spring, 6500 fl., House Mtn., Millard Co., Utah”. Known only from Millard County, western Utah.
63. M. f. solitarius (Grinnell, 1926). Type locality “Fingerrock Wash, 5400 ft., Stewart Valley, Mineral Co., Nevada”. Known only from Mineral County, western Nevada.
64. M. f. stansburyi (Durrant, 1946). Type locality “South Willow Creek, Stansbury Mts., 7500 ft., Tooele Co., Utah”. Known only from Tooele County, northwestern Utah.
65. M. f. subsimilis (Goldman, 1933). Type locality “Harquahala Mt., 3000 ft., Yuma Co., Arizona”. Known only from Yuma County, southwestern Arizona.
66. M. f. tivius (Durrant, 1937). Type locality “Oak Creek Canyon, 6 mi. E Oak City, 6000 ft., Millard Co., Utah”. Known only from Millard County, western Utah.
67. M. f. toltecus (Allen, 1893). Type locality “Colonia Juarez, 4500 ft., Casas Grandes River, Chihuahua”. Range from southern New Mexico and northern Chihuahua.
Megascapheus laticeps (Baird, 1855)
Northern California pocket gopher, tuza del norte de California
1855. Thomomys laticeps Baird, Proc. Acad. Nat. Sci. Philadelphia, 7:335. Type locality. Humboldt Bay, Humboldt Co., California.
2024. Megascapheus laticeps: (this study).
1. M. l. agricolaris (Grinnell, 1935). Type locality “Stralock Farm, 3 mi. W Davis, Yolo Co., California”. Known only from Yolo County, California.
2. M. l. awahnee (Merriam, 1908). Type locality “Yosemite Valley, 4000 ft., near old Sentinel Hotel, Mariposa Co., California”. Range in southern Sierra Nevada, California.
3. M. l. detumidus (Grinnell, 1935). Type locality “1 1/2 mi. S (town of) Pistol River, 250 ft., Curry Co., Oregon”. Known only from Curry County, Oregon.
4. M. l. laticeps (Baird, 1855). For type locality see above. Range from Humboldt County, California north through southern Oregon.
5. M. l. leucodon (Merriam, 1897). Type locality “Grant Pass, Rogue River Valley, Oregon”. Range in highlands around the northern Central Valley, California, and southwestern Oregon.
6. M. l. saxatilis (Grinnell, 1934). Type locality “1 mi. N Susan·ville, 4400 ft., Lassen Co., California”. Known only from Lassen County, California.
Megascapheus nayarensis (Mathis et al., 2013)
Nayarit pocket gopher, tuza del Nayar
2013. Thomomys nayarensis Mathis, Hafner, Hafner, and Demastes; Jour. Mamm 94:989. Type locality. 8.5 km N, 7 km W Mesa del Nayar (formerly listed by Hafner et al. [2011] as ‘’22 km S, 3 km E Santa Teresa), 2,200 m (22.290, _104.721), Nayarit, México”.
2024. Megascapheus nayarensis: (this study).
Megascapheus nigricans (Rhoads, 1895)
California pocket gopher, tuza de Baja California
1895. Thomomys fulvus nigricans Rhoads; Proc. Acad. Nat. Sci. Philadelphia, 47:36. Type locality. Witch Creek, 2753 ft., 7 mi. W Julian, San Diego Co., California.
2013. Thomomys nigricans: Trujano-Álvarez and Álvarez-Castañeda, Zool. Jour. Linn, Soc. 168:886.
2024. Megascapheus nigricans: (this study).
1. M. n. anitae (Allen, 1898). Type locality “Santa Anita, Baja California [Sur]”. Range from southern Vizcaíno Desert south to the southern tip of Baja California Peninsula.
2. M. n. martirensis (Allen, 1898). Type locality “La Grulla Meadow, Sierra San Pedro Mártir, 7400 ft., Baja California”. Range from Sierra Juárez south through the Central Desert, Baja California.
3. M. n. nigricans (Rhoads, 1895). For type locality see above. Known only from southern California and northwestern Baja California.
4. M. n. russeolus (Nelson and Goldman, 1909). Type locality “San Angel, WSW San Ignacio, Baja California”. Known only from the Vizcaíno Desert, northern Baja California Sur, and southern Baja California.
Megascapheus ruidosae (Hall, 1932)
Ruidoso pocket gopher, tuza de Ruidoso
1932. Thomomys umbrinus ruidosae Hall, Proc. Biol. Soc. Washington, 45:96. Type locality “Ruidoso, 6700 ft., Lincoln Co., New Mexico.
2024. Megascapheus ruidosae: (this study).
Megascapheus sheldoni (Bailey, 1915)
Sheldon´s pocket gopher, tuza de la Sierra Madre
1915. Thomomys sheldoni V. Bailey, N. Amer. Fauna, 39:93, November 15. Type locality “Santa Teresa, 6800 ft., Sierra del Nayarit, Nayarit”.
2024. Megascapheus sheldoni: (this study).
1. M. s. chihuahuae (Nelson and Goldman, 1934). Type locality “Sierra Madre, 7000 ft., about 65 mi. E Batopilas, Chihuahua”. Known only from the Sierra Madre Occidental highlands, Chihuahua.
2. M. s. sheldoni (Bailey, 1915). For type locality see above. Known only from the Sierra Madre Occidental highlands, western Durango, northeastern Nayarit, and western Zacatecas.
Megascapheus townsendii (Bachman, 1839)
Townsend´s pocket gopher, tuza de las montañas del oeste
1839. Geomys townsendii Bachman, Jour. Acad. Nat. Sci. Philadelphia, 8:105. Type locality. Erroneously given as "Columbia River," but probably near Nampa, Canyon Co., Idaho, where Townsend's party camped to trade with Indians, August 22, 1834 (V. Bailey, N. Amer. Fauna, 39:42, November 15, 1915).
1968. Thomomys townsendii: Thaler, Univ. California Pub. Zool. 86.
2024. Megascapheus townsendii: (this study).
1. M. t. nevadensis (Merriam, 1897). Type locality “Reese River Valley, 5 mi. W Austin, Lander Co., Nevada”. Central-northern Nevada, southeastern Oregon, and California.
2. M. t. townsendii (Bachman, 1839). For type locality see above. Known only from Snake River, western Idaho, and eastern Oregon.
Megascapheus umbrinus (Richardson, 1829)
Southern pocket gopher, tuza mexicana
1829. Geomys umbrinus Richardson, Fauna Boreali-Americana,1:202. Type locality “southern México; probably vic. Boca del Monte, Veracruz”; type said to have come from "Cadadaguois, a town in southwestern Louisiana"; see V. Bailey, Proc. Biol. Soc. Washington, 19:3-6, January 29, 1906.
1855. Thomomys umbrinus: Baird, Proc. Acad. Nat. Sci. Philadelphia, 7:332.
2024. Megascapheus umbrinus: (this study).
1. M. u. durangi (Nelson and Goldman, 1934). Type locality “Durango, Durango”. Range from southwestern Durango to extreme northwestern Zacatecas.
2. M. u. goldmani (Merriam, 1901). Type locality “Mapimi, 3800 ft., Durango”. Range in central Chihuahua south through central-eastern Durango and southwestern Coahuila.
3. M. u. intermedius (Mearns, 1897). Type locality “summit Huachuca Mts., 9000 ft., Arizona”. Range from southeastern Arizona south through Sonora and northwestern Chihuahua.
4. M. u. umbrinus (Richardson, 1829). For type locality see above. Range from eastern-central Zacatecas south through the Eje Neovolcánico in Veracruz.
Thomomys Wied-Neuwied, 1839
1839. Thomomys Wied-Neuwied, Nova Acta Phys.-Med. Acad. Caesar. Leop.-Carol., 19(pt. 1):377. Type species. Thomomys rufescens Wied-Neuwied, 1839.
Content. Five allopatric species of Thomomys are recognized: Thomomys clusius Coues, 1875, Thomomys idahoensis Merriam, 1901, Thomomys mazama Merriam, 1897, Thomomys monticola J. A. Allen, 1893, and Thomomys talpoides (Richardson, 1828).
Etymology. The name Thomomys is derived from the Greek thomos, meaning “pile”, and mys, “mouse”: related to the pile of earth accumulated at the entrance of its burrows.
Diagnosis. The species of the genus Thomomys can be distinguished from those of Megascapheus by having the upper incisors no procumbent and their root above the fourth upper premolars; sphenoidal fissure close (except for some specimens of T. clusius); angular process not continuous, with a weakly-developed flange along the ventral ramus side; rostrum slender; base of the first lower premolars nearly perpendicular to the occlusal surface of the toothrow; infraorbital canals opening directly above or slightly posterior to the incisive foramina; anterior enamel plate of the first lower premolars recurved, frequently forming a shallow re-entrant angle; anterior enamel plate of the first lower premolars broad and only slightly separated from the posterior enamel plate (rarely continuous), with the lateral enamel plate on the lingual side; diploid chromosome numbers from 40 to 60 (Nelson and Goldman 1934; Russell 1968a; Álvarez-Castañeda 2024).
Distribution. Thomomys ranges from southern British Columbia, Alberta, Saskatchewan, and Manitoba southward through California, Nevada, Arizona, and New Mexico; eastward through North Dakota, South Dakota, Nebraska, and Colorado; and from Washington, Oregon, and California east through Manitoba, eastern North Dakota, and South Dakota, southwestern Nebraska, eastern Colorado, and central New Mexico.
Comments. Thaeler (1980) suggests that the current T. talpoides could be split into 10–12 separate species. The genetic data show that T. talpoides is a species complex and should be reviewed in detail.
Thomomys clusius Coues, 1875
Wyoming pocket gopher, tuza de Wyoming
1875. Thomomys clusius Coues, Proc. Acad. Nat. Sci. Philadelphia 27:138. Type locality “Bridger Pass, 18 mi. SW Rawlins, Carbon Co., Wyoming”.
Thomomys idahoensis Merriam, 1901
Idaho pocket gopher, tuza de Idaho
1901. Thomomys idahoensis Merriam, Proc. Biol. Soc. Washington, 14:114. Type locality “Brich Creek, Clark Co., Idaho”.
1. T. i. confinis Davis, 1937. Type locality “Gird Creek, near Hamilton, Ravalli Co., Montana”. Known only from Ravalli County, Montana.
2. T. i. idahoensis Merriam, 1901. For type locality see above. Range in southeastern Idaho and southwestern Montana.
3. T. i. pygmaeus Merriam, 1901. Type locality “Montpelier Creek, 6700 ft., about 10 mi. NE Montpelier, Bear Lake Co., Idaho”. Range in southwestern Wyoming and southeastern Idaho.
Thomomys mazama Merriam, 1897
Western pocket gopher, tuza del oeste
1897. Thomomys mazama Merriam, Proc. Biol. Soc. Washington, 11:214. Type locality “Anna Creek, 6000 ft., near Crater Lake, Mt. Mazama, Klamath Co., Oregon”.
1. T. m. couchi Goldman, 1939. Type locality “4 mi. N Shelton, Mason Co., Washington”. Known only from Mason County, Washington.
2. T. m. glacialis Dalquest and Scheffer, 1942. Type locality “prairie 2 mi. S Roy, Pierce Co., Washington”. Known only from Pierce County, Washington.
3. T. m. helleri Elliot, 1903. Type locality “Goldbeach, mouth of Rogue River, Curry Co., Oregon”. Known only from Curry County, Oregon.
4. T. m. hesperus Merriam, 1901. Type locality “Tillamook, Tillamook Co., Oregon”. Range in coastal area of northwestern Oregon.
5. T. m. louiei Gardner, 1950. Type locality “12 mi, NNE Cathlamet (Crown-Zellerbach's Cathlamet Tree Farm), 2500 ft., Wahkiakum Co., Washington”. Known only from Wahkiakum County, Washington.
6. T. m. mazama Merriam, 1897. For type locality see above. Range in central-western Oregon and northern California.
7. T. m. melanops Merriam, 1899. Type locality “timberline at head Soledue River, Olympic Mts., Clallam Co., Washington”. Range in northern Olympia peninsula, Washington.
8. T. m. nasicus Merriam, 1897. Type locality “Farewell Bend, Deschutes River, Deschutes Co., Oregon”. Known only from central-western Oregon.
9. T. m. niger Merriam, 1901. Type locality “Seaton (= Mapleton), near mouth Umpqua River (= head tidewater, Siuslaw River), Lane Co., Oregon”. Known only from Benton and Lane Counties, Oregon.
10. T. m. oregonus Merriam, 1901. Type locality “Ely, near Oregon City, Willamette Valley, Clackamas Co., Oregon”. Known only from northwestern Oregon.
11. T. m. premaxillaris Grinnell, 1914. Type locality “2 mi. S South Yolla Bolly Mtn., 7500 ft., Tehama Co., California”. Known only from Tehama County, California.
12. T. m. pugetensis Dalquest and Scheffer, 1942. Type locality “3 mi. S Olympia, Thurston Co., Washington”. Known only from Thurston County, Washington.
13. T. m. tacomensis Taylor, 1919. Type locality “6 mi. S Tacoma, Pierce Co., Washington”. Known only from Tacoma County, Washington.
14. T. m. tumuli Dalquest and Scheffer, 1942. Type locality “7 mi. N Tenino, Thurston Co., Washington”. Known only from Thurston County, Washington.
15. T. m. yelmensis Merriam, 1899. Type locality “Tenino, Yelm Prairie, Thurston Co., Washington”. Known only from Thurston County, Washington.
Thomomys monticola J. A. Allen, 1893
Mountain pocket gopher, tuza de las montañas
1893. Thomomys monticola J. A. Allen, Bull. Amer. Mus. Nat. Hist., 5:48. Type locality “Mt. Tallac, 7500 ft., El Dorado Co., California”.
Thomomys talpoides (Richardson, 1828)
Northern pocket gopher, tuza del norte
1828. Cricetus talpoides Richardson, Zool. Jour., 3:518. Type locality “Fixed at near Fort Carlton (Carlton House), Saskatchewan River, Saskatchewan, Canada”.
1858. Thomomys talpoides: Baird, Mammals, in Repts. Expl. Surv .... , 8(1):403.
1. T. t. aequalidens Dalquest, 1942. Type locality “Abel Place, 2200 ft., 6 mi. SSE Dayton, Columbia Co., Washington”. Known only from southeastern Oregon.
2. T. t. agrestis Merriam, 1908. Type locality “Medano Ranch, San Luis Valley, Colorado”. Known only from southern-central Colorado.
3. T. t. andersoni Goldman, 1939. Type locality “Medicine Hat, South Saskatchewan River, Alberta”. Known only from southwestern Alberta.
4. T. t. attenuatus Hall and Montague, 1951. Type locality “3½ mi. W Horse Creek P.O., 7000 ft., Laramie Co., Wyoming”. Range in southeastern Wyoming and northeastern Colorado.
5. T. t. bridgeri Merriam, 1901. Type locality “Harvey's Ranch, Smith Fork, 6 mi. SW Old Fort Bridger, Uinta Co., Wyoming”. Range in southeastern Idaho, western and southwestern California.
6. T. t. bullatus Bailey, 1914. Type locality “Powderville, Custer Co., Montana”. Range in southern Saskatchewan, eastern Montana, and northeastern Wyoming.
7. T. t. caryi Bailey, 1914. Type locality “head Trapper Creek, 9500 ft., Bighorn Mts., Bighorn Co., Wyoming”. Range in central-northern Wyoming and southern-central- Montana.
8. T. t. cheyennensis Swenk, 1941. Type locality “2 mi. S Dalton, Cheyenne Co., Nebraska”. Known only from western Nebraska.
9. T. t. cognatus Johnstone, 1955. Type locality “Crowsnest Pass, British Columbia”. Known only from southeastern British Columbia.
10. T. t. columbianus Bailey, 1914. Type locality “Touchet, Walla Walla Co., Washington”. Known only from northern-central Oregon.
11. T. t. devexus Hall and Dalquest, 1939. Type locality “1 mi. WSW Neppel, Grant Co., Washington”. Known only from central-southeastern Washington.
12. T. t. douglasii (Richardson, 1829). Type locality “near mouth Columbia River, probably near Vancouver, Washington”. Known only from southwestern Washington.
13. T. t. durranti Kelson, 1949. Type locality “Johnson Creek, 14 mi. N Blanding, 7500 ft., San Juan Co., Utah”. Range in eastern Idaho and western-central Colorado.
14. T. t. falcifer Grinnell, 1926. Type locality “Bells Ranch, 6890 ft., Reese River Valley, Nye Co., Nevada”. Known only from central Nevada.
15. T. t. fisheri Merriam, 1901. Type locality “Beckwith, Sierra Valley, Plumas Co., California”. Range in western California and eastern Nevada.
16. T. t. fossor Allen, 1893. Type locality “Florida, 7200 ft., La Plata Co., Colorado”. Range in northeastern Arizona, southwestern Colorado, and northern New Mexico.
17. T. t. fuscus Merriam, 1891. Type locality “Summit Creek in mountains, head Big Lost River, Custer Co., Idaho”. Known only from central Idaho.
18. T. t. gracilis Durrant, 1939. Type locality “Pine Canyon, 6600 ft., 17 mi. NW Kelton, Boxelder Co., Utah”. Range in northwestern Idaho, northern, central, and northeastern Nevada.
19. T. t. immunis Hall and Dalquest, 1939. Type locality “5 mi. S Trout Lake, Klickitat Co., Washington”. Known only from central-southern Washington.
20. T. t. incensus Goldman, 1939. Type locality “Shuswap, Yale District, British Columbia”. Known only from southern British Columbia.
21. T. t. kaibabensis Goldman, 1938. Type locality “De Motte Park, 9000 ft., Kaibab Plateau, Coconino Co., Arizona”. Known only from northern-central Arizona.
22. T. t. kelloggi Goldman, 1939. Type locality “West Boulder Creek, Absaroka Mts., 18 mi. SE Livingston, Park Co., Montana”. Known only from Park County, Montana.
23. T. t. levis Goldman, 1938. Type locality “Seven Mile. Flat, 10,000 ft., 5 mi. N Fish Lake, Fish Lake Plateau, Sevier Co., Utah”. Known only from central Idaho.
24. T. t. limosus Merriam, 1901. Type locality “White Salmon, Gorge of the Columbia, Klickitat Co., Washington”. Known only from southern Washington.
25. T. t. loringi Bailey, 1914. Type locality “South Edmonton, Alberta”. Known only from central-western Alberta.
26. T. t. macrotis Miller, 1930. Type locality “D' Arey Ranch, 2 mi. N Parker, Douglas Co., Colorado”. Known only from Douglas County, Colorado.
27. T. t. medius Goldman, 1939. Type locality “Silver King Mine, summit Toad Mtn., 6 mi. S Nelson, West Kootenay District, British Columbia”. Known only from southeastern British Columbia.
28. T. t. meritus Hall, 1951. Type locality “8 mi. N, 19½ mi. E Savery, 8800 ft., Carbon Co., Wyoming”. Known only from central-northern Colorado.
29. T. t. monoensis Huey, 1934. Type locality “Dexter Creek Meadow, 6800 ft., at confluence Dexter and Wet creeks, Mono Co., California”. Range in eastern California and western Nevada.
30. T. t. moorei Goldman, 1938. Type locality “1 mi. S Fairview, 6000 ft., Sanpete Co., Utah”. Known only from central Utah.
31. T. t. nebulosus Bailey, 1914. Type locality “Jack Boyden's Ranch, 3750 ft., Sand Creek Canyon, 15 mi. NE Sundance, Crook Co., Wyoming”. Range in western South Dakota and eastern Wyoming.
32. T. t. ocius Merriam, 1901. Type locality “Mountainview, Smiths Fork, 4 mi. (by airline) SE Fort Bridger, Uinta Co., Wyoming”. Range in southwestern Wyoming, northwestern Colorado, and northeastern Utah.
33. T. t. oquirrhensis Durrant, 1939. Type locality “Settlement Creek, 6500 ft., Oquirrh Mts., Tooele Co., Utah”. Known only from central-northern Utah.
34. T. t. parowanensis Goldman, 1938. Type locality “Brian Head, 11,000 ft., Parowan Mts., Iron Co., Utah”. Known only from southern Utah.
35. T. t. pierreicolus Swenk, 1941. Type locality “Wayside, Dawes Co., Nebraska”. Known only from western Nebraska.
36. T. t. pryori Bailey, 1914. Type locality “head Sage Creek, 6000 ft., Pryor Mts., Carbon Co., Montana”. Known only from southern Montana.
37. T. t. quadratus Merriam, 1897. Type locality “The Dalles, Wasco Co., Oregon”. Known only from southeastern Oregon.
38. T. t. ravus Durrant, 1946. Type locality “19 mi. N Vernal, 8000 ft., Uintah Co., Utah”. Known only from northeastern Utah.
39. T. t. relicinus Goldman, 1939. Type locality “Twin Springs, 20 mi. N Minidoka, Snake River Desert, Minidoka Co., Idaho”. Known only from central-southern Idaho.
40. T. t. retrorsus Hall, 1951. Type locality “Flagler, Kit Carson Co., Colorado”. Known only from eastern Colorado.
41. T. t. rostralis Hall and Montague, 1951. Type locality “1 mi. E Laramie, 7164 ft., Albany Co., Wyoming”. Range in southern-central Wyoming and central Colorado.
42. T. t. rufescens Wied-Neuwied, 1839. Type locality “Minnetaree Village, now Old Fort Clark, about 6 mi. S Stanton, Mercer Co., North Dakota”. Range in southwestern Saskatchewan and Manitoba south through South Dakota.
43. T. t. saturatus Bailey, 1914. Type locality “Silver, near Saltese, Coeur d'Alene Mts., Missoula Co., Montana”. Range in northern Idaho, northwestern Montana, and southeastern British Columbia.
44. T. t. segregatus Johnstone, 1955. Type locality “Goat Mtn., E side Kootenay River, near Wynndel, British Columbia”. Known only from near Wynndel, southeastern British Columbia.
45. T. t. shawi Taylor, 1921. Type locality “Owyhigh Lake, 5100 ft., Mt. Rainier, Pierce Co., Washington”. Range in central-southern Washington.
46. T. t. talpoides (Richardson, 1828). For type locality see above. Range in western Alberta, central Saskatchewan, central-western Manitoba, and northern-central Montana.
47. T. t. taylori Hooper, 1940. Type locality “6 mi. NE summit Mt Taylor, about 8900 ft., near Fernandez summer camp, Valencia Co., New Mexico”. Known only from Valencia County, central New Mexico.
48. T. t. tenellus Goldman, 1939. Type locality “Whirlwind Peak, 10,500 ft., Absaroka Range, Park Co., Wyoming”. Range in northwestern Wyoming and southern Montana.
49. T. t. trivialis Goldman, 1939. Type locality “near head Big Timber Creek, 5200 ft., about 15 mi. NW Big Timber, Crazy Mts., Sweetgrass Co., Montana”. Known only from central Montana.
50. T. t. uinta Merriam, 1901. Type locality “Blacks Fork, 10,000 ft., N base Gilbert Peak, Uinta Mts., Summit Co., Utah”. Known only from central-northern Utah.
51. T. t. wallowa Hall and Orr, 1933. Type locality “Catherine Creek, 3500 ft., 7 mi. E Telocaset, Union Co., Oregon”. Known only from northeastern Oregon.
52. T. t. wasatchensis Durrant, 1946. Type locality “Midway, 5500 ft., Wasatch Co., Utah”. Known only from northern Utah.
53. T. t. whitmani Drake and Booth, 1952. Type locality “Whitman National Monument, 750 ft., 6 mi. W Walla Walla, Walla Walla Co., Washington”. Known only from Walla Walla County, eastern Washington.
54. T. t. yakimensis Hall and Dalquest, 1939. Type locality “Selah, Yakima Co., Washington”. Range in central-southern Washington.
Tribe Geomyini (Clade B)
Sub-clade B1
Genus Cratogeomys Merriam, 1895
1895. Cratogeomys Merriam, N. Amer. Fauna, 8:150. Type species. Geomys merriami (Thomas, 1893).
Content. Seven allopatric species are recognized species of Cratogeomys are recognized: Cratogeomys castanops (Baird, 1852), Cratogeomys fulvescens Merriam, 1895, Cratogeomys fumosus (Merriam, 1892), Cratogeomys goldmani Merriam, 1895, Cratogeomys merriami (Thomas, 1893), Cratogeomys perotensis Merriam, 1895, and Cratogeomys planiceps (Merriam, 1895).
Etymology. The name Cratogeomys is derived from the Greek krataios, meaning “strong”; geo, "earth"; and mys, “mouse”: strong earth mouse, referring to strong and large specimens within the gophers (Jaeger 1955).
Diagnosis. Dorsal pelage in different colors, mainly shades of brown; fur of underparts paler than in the dorsum; tail short and slightly darker dorsally; body compact and cylindrical; hair silky and long, approximately 8.0 mm; skull large and massive; no ridge on the squamosal but a sagittal crest mainly in adult and old males; interorbital region narrower than the rostrum; zygomatic lateral angle with a plate-shaped expansion. The genus Cratogeomys can be differentiated from other species of the family Geomyidae by the following characteristics: rostrum wider than the interorbital region, zygomatic without an anterior angle plate-shaped expansion, and third upper molars not clearly showing two lobes (Merriam 1895; Nelson and Goldman 1934; Russell 1968a; Álvarez-Castañeda 2024).
Distribution. The species of Cratogeomys range from Colorado, Kansas, Oklahoma, New Mexico, and Texas southward throughout the Altiplano Mexicana to the Eje Volcánico Trasversal the Oriental Basin, eastern Puebla, western Veracruz, and western Tlaxcala throughout Jalisco, Colima, and Nayarit (Russell 1968a, Hall 1981; Hafner et al. (2004, 2005, 2008). All species of Cratogeomys have allopatric distribution.
Comments. Revisions of the species in the genus Cratogeomys have been performed by Russell (1968b) and Hafner et al. (2004, 2005, 2008). The genus Cratogeomys comprises two species groups: castanops (C. castanops, C. fulvescens, C. goldmani, C. merriami, and C. perotensis) and fumosus (C. fumosus and C. planiceps; Hafner et al. 2004). The revision of C. castanops by Hafner et al. (2008) considered that it should be split into two different species, with the boundary between the species located at the Nazas River and Sierra de Parras. C. castanops is distributed north of this boundary and C. goldmani ranges to the south; all other subspecies should be considered junior synonyms. C. castanops castanops includes C. c. angusticeps, C. c. bullatus, C. c. convexus C. c. dalquesti C. c. hirtus, C. c. parviceps, C. c. perplanus, C. c. pratensis, C. c. simulans, C. c. tamaulipensis, C. c. torridus, and C. c. ustulatus as junior synonyms. The subspecies C. c. consitus includes the subspecies C. c. excelsus, C. c. goldmani (part of the distribution), C. c. jucundus, C. c. perexiguus, C. c. sordidulus, C. c. subsimus, and C. c. surculus (part of the distribution). C. goldmani is distributed south of the Nazas River and Sierra de Parras, and comprises two subspecies: C. g. goldmani, which includes the subspecies of C. castanops: C. c. goldmani as a junior synonym (part of the distribution), C. c. rubellus, and C. c. surculus (part of the distribution). The subspecies C. g. subnubilus includes the subspecies C. c. subnubilus, C. c. elibatus, C. c. maculatus, C. c. peridoneus, and C. c. planifrons. The populations previously considered subspecies of C. merriami (Russell 1968b), namely C. m. perotensis and C. m. fulvescens, were elevated to full species (Hafner et al. 2005). On the other hand, C. merriami estor, C. m. peraltus, C. m. irolonis, and C. m. saccharalis are not considered valid subspecies, the first are junior synonyms of C. perotensis and the later of C. merriami (Hafner et al. 2005). Cratogeomys fumosus angustirostris, C. f. imparilis and C. f. tylorhinus were considered subspecies of C. fumosus. C. f. angustirostris comprises Pappogeomys zinseri and P. tylorhinus brevirostris as junior synonyms. C. f. fumosus includes P. gymnurus gymnurus, P. g. inclarus, P. g. tellus, P. tylorhinus atratus, P. t. zodius, and C. zinseri morulus; C. f. tylorhinus includes P. neglectus and C. t. arvalis (Hafner et al. 2004).
The morphological differences that differentiate Pappogeomys from Cratogeomys include the tail usually naked and less than one-half of the head-and-body length vs tail short and slightly darker dorsally; claws on the forefeet larger vs smaller; interorbital region wider vs narrower than the rostrum; sagittal crest absent vs present; zygomatic without an anterior angle and plate-shaped expansion vs lateral angles with a plate-shaped expansion (Merriam 1895; Nelson and Goldman 1934; Russell 1968a; Álvarez-Castañeda 2024). The characteristics that differentiate Pappogeomys from Orthogeomys include size less than 350 mm vs size greater than 350.0 mm; body mass less than 600 g vs greater than 800 g); third upper molars clearly with two lobes, outer lingual angle well developedvs third upper molars not clearly with two lobes, outer lingual angle not well developed; upper premolars usually with an enamel plate on the back restricted to the lingual region vs upper premolars never with an enamel plate in the lingual region (Russell 1968a; Hall 1981; Álvarez-Castañeda 2024). The characteristics listed above are easily observed and useful for separating Pappogeomys from Cratogeomys and Ortogeomys, as many of the authors cited previously have demonstrated.
Cratogeomys castanops (Baird, 1852)
Yellow-faced pocket gopher, tuza de cara amarilla
1852. Pseudostoma castanops Baird, in Rept. Stan bury' s Expl. Surv. ... Great Salt Lake of Utah... , App. C, p. 313. Type locality “prairie road to Bent's Fort, near present town of Las Animas, Bent Co., Colorado”.
1985. Cratogeomys castanops: Merriam, N. Amer. Fauna, 8:159.
1. C. c. castanops (Baird, 1852). For type locality see above. Range from northern Tamaulipas, Coahuila, and Chihuahua north through Colorado, Kansas, Oklahoma, New Mexico, and Texas.
2. C. c. consitus Nelson and Goldman, 1934. Type locality “Gallego, 5500 ft., Chihuahua”. Range from northern Tamaulipas, Coahuila, Chihuahua, and Sonora south through the Nazas River and Sierra de Parras, Coahuila, and Durango”.
Cratogeomys fulvescens Merriam, 1895
Oriental Basin pocket gopher, tuza de la Cuenca Oriental
1895. Cratogeomys fulvescens Merriam, N. Amer. Fauna, 8:161. Type locality “Chalchicomula [= Ciudad Serdán), 8200 ft., Puebla”.
Cratogeomys fumosus (Merriam, 1892)
Smoky pocket gopher, tuza del centro de México
1892. Geomys fumosus Merriam, Proc. Biol. Soc. Washington, 7:165. Type locality “3 mi. W Colima, 1700 ft., Colima”.
1948. Cratogeomys fumosus: Hooper, Jour. Mamm., 29:302.
1. C. f. angustirostris (Merriam, 1903). Type locality “Cerro Patambán, 10,000 ft., Michoacán”. Known only from the southwestern central Mexican Plateau.
2. C. f. fumosus (Merriam, 1892). For type locality see above. Patchily distributed on western Michoacán, eastern slopes of the Sierra Madre del Sur in Jalisco and Colima.
3. C. f. imparilis (Goldman, 1939). Type locality “Pátzcuaro, Michoacán”. Patchily distributed in central Michoacán.
4. C. f. tylorhinus (Merriam, 1895). Type locality “Tula, 6800 ft., Hidalgo”. Patchily distributed across the southeastern central Mexican Plateau.
Cratogeomys goldmani Merriam, 1895
Goldman`s pocket gopher, tuza del Altiplano
1895. Cratogeomys castanops goldmani Merriam, N. Amer. Fauna, 8:160. Type locality “Cañitas, Zacatecas”.
1987. Cratogeomys goldmani: Lee and Baker, Occ. Pap., Mus. Texas Tech Univ. 114:13.
1. C. g. goldmani Merriam, 1895. For type locality see above. Ranges from Nazas River and Sierra de Parras, Coahuila, and Durango south through San Luis Potosí and Zacatecas.
2. C. g. subnubilus Nelson and Goldman, 1934. Type locality “Cameros, Coahuila”. Range from Monterrey, Nuevo León, along western Tamaulipas, and San Luis Potosí.
Cratogeomys merriami (Thomas, 1893)
Merriam’s pocket gopher, tuza del Valle de México
1893. Geomys merriami Thomas, Ann. Mag. Nat. Hist., ser. 6, 12:271. Type locality “southern Mexico (Probably the Valley of Mexico according to Merriam, N. Amer. Fauna, 8:152, 1895.)”
1982. Cratogeomys merriami: Honeycutt and Williams, Jour. Mamm. 63:212.
Cratogeomys perotensis Merriam, 1895
Perote pocket gopher, tuza de Perote
1895. Cratogeomys perotensis Merriam, N. Amer. Fauna, 8:154. Type locality “Cofre de Perote, 9500 ft., Veracruz”.
Cratogeomys planiceps (Merriam, 1895)
Toluca Volcano pocket gopher, tuza del Nevado de Toluca
1895. Platygeomys planiceps Merriam, N. Amer. Fauna, 8:168. Type locality “N slope Volcán de Toluca, 9000 ft., México”.
2004. Cratogeomys planiceps: Hafner, Spradling, Light, Hafner, and Demboski. Jour. Mamm 85:1178.
Orthogeomys Merriam, 1895
1895. Orthogeomys Merriam, N. Arner. Fauna, 8:172. Type species. Geomys scalops (Thomas, 1894).
Content. Only one species of Orthogeomys is recognized: O. grandis (Thomas, 1893).
Etymology. The name Orthogeomys is derived from the Greek orthos, meaning “straight”; geo, "earth"; and mys, “mouse”. The name refers to the unusual shape of the skull (Merriam 1895).
Diagnosis.— Orthogeomys has the largest members of the family Geomyidae; dorsal pelage reddish cinnamon to brownish; fur of underparts paler; body compact and cylindrical; hair sparse and bristly; skull large and massive; ridge on the squamosal joining the temporal in adult and old males; rostrum narrower than the interorbital region, no interorbital constriction; frontal bone wide and inflated; anterior surface of the upper incisors with one deep groove; last upper molars semi-lobular, only one labial groove; an enamel plate covering the front and re-entrant angle edge of the first upper and lower molariforms; first lower molariforms with an enamel plate and first upper molariforms without a small plate on the lingual side; fourth upper premolars without an enamel plate, although a small plate restricted to the lingual end of the wall rarely present (Russell 1968a; Hall 1981; Álvarez-Castañeda 2024).
Distribution. Patchily distributed along the Pacific coast Colima and Jalisco southward to southwestern Honduras, including Guatemala and El Salvador; altitudinal range from near sea level to at least 2,700 m (Hall 1981; Spradling et al. 2016).
Comments. Orthogeomys ciniculus was considered as a valid species, but the genetic analyses with nDNA and mtDNA show the absence of reciprocal monophyly. Given this finding, it is considered a junior synonym of O. grandis.
Orthogeomys grandis (Thomas, 1893)
Giant pocket gopher, tuza gigante del Pacífico
1893. Geomys grandis Thomas, Ann. Mag. Nat. Hist., ser. 6, 12:270. Type locality “Dueñas, Guatemala”.
1895. Orthogeomys grandis, Merriam, N. Amer. Fauna, 8:175.
1. O. g. alleni Nelson and Goldman, 1930. Type locality “near Acapulco, Guerrero, Mexico (altitude 2,000 feet)”. Range in southeastern Jalisco coastal plains through central Oaxaca.
2. O. g. alvarezi Schaldach, 1966. Type locality “ridge above Lachao (pass above Kilometer 183), on road from Oaxaca City to Puerto Escondido, approximately 40 kms. N. San Gabriel Mixtepec, Municipio de Juquila, Oaxaca, Mexico, altitude approximately 1700 m”. Known only from San Gabriel Mixtepec, Oaxaca.
3. O. g. annexus Nelson and Goldman, 1933. Type locality “Tuxtla Gutierrez, 2600 ft., Chiapas”. Known only from Tuxtla Gutiérrez.
4. O. g. carbo Goodwin, 1956. Type locality “Escurano, 2500 ft., Cerro de San Pedro, 20 km W Mixtequilla, Oaxaca [Mexico]”. Known only from the central coast of Oaxaca.
5. O. g. cuniculus Elliot, 1905. Type locality “Zanatepec, Oaxaca”. Known only from type locality.
6. O. g. engelhardi Felten, 1957. Type locality “Finca El Carmen (1,319 m), Volcán de San Vicente, [San Vicente Department] El Salvador”. Known only from type locality.
7. O. g. felipensis Nelson and Goldman, 1930. Type locality “Cerro San Felipe, 10 miles north of Oaxaca, Oaxaca, Mexico (altitude 10,000 feet)”. Known only from the Central Valleys of Oaxaca.
8. O. g. grandis (Thomas, 1893). Type see above. Known only from the volcano arch of Guatemala.
9. O. g. guerrerensis Nelson and Goldman, 1930. Type locality “El Limon, in the valley of the Rio de las Balsas about 20 miles northwest of La Union, Guerrero [Mexico]”. Known only from the central lowlands of Guerrero.
10. O. g. huixtlae Villa R., 1944. Type locality “Finca Lubeca, 12 km NE Huixtla, 850 m, Chiapas [Mexico]”. Known only from southeastern Chiapas.
11. O. g. latifrons Merriam, 1895. Type locality “Guatemala (exact locality unknown, but probably lowlands of southern Guatemala)”. Known only from Guatemala.
12. O. g. nelsoni Merriam, 1895. Type locality “Mt. Zempoaltepec, 8000 ft., Oaxaca”. Known only from Sierra Norte of Oaxaca.
13. O. g. pluto Lawrence, 1933. Type locality “Cerro Cantoral, north of Tegucigalpa, Honduras”. Known only from type locality.
14. O. g. pygacanthus Dickey, 1928. Type locality “Cacaguatique, 3500 ft. Department of San Miguel, El Salvador”. Known only from western and central El Salvador.
15. O. g. scalops (Thomas, 1894). Type locality “Tehuantepec, Oaxaca”. Known only from the Isthmus of Tehuantepec, Oaxaca.
16. O. g. soconuscensis Villa R., 1949. Type locality “Finca Esperanza, 710 m, 45 km (by road) NW Huixtla, Chiapas [Mexico]”. Known only from the coast of Chiapas.
17. O. g. vulcani Nelson and Goldman, 1931. Type locality “Volcan Santa Maria, Quezaltenango, Guatemala (altitude 9,000 feet)”.
Genus Pappogeomys Merriam, 1895
1895. Pappogeomys Merriam, N. Amer. Fauna, 8:145. Type species. Geomys bulleri (Thomas, 1892).
Content. One species of Pappogeomys is recognized: Pappogeomys bulleri (Thomas, 1892).
Etymology. The name Pappogeomys is derived from the Greek pappos, meaning “grandfather”; geo, "earth", and mys, “mouse”: the grandfather of the earth mouse (Jaeger 1955).
Diagnosis. Buller’s pocket gopher is medium-sized; total length 200.0 mm to 249.0 mm, skull length 39.2 mm to 42.9 mm. Pelage medium in length and soft, except in coastal populations which have short and sparse pelage; color light brown to dark gray; fur of underparts paler than in the dorsum; most specimens with a small nasal patch consisting of white or pale buffy hairs; body compact and cylindrical; tail usually naked and less than one-half of the head-and-body length; claws on forefeet larger than in Cratogeomys; skull large and massive; rostrum wider than the interorbital region; incisors without grooves in the frontal phase; without a zygomatic anterior angle with a plate-shaped expansion (Merriam 1895; Nelson and Goldman 1934; Russell 1968a; (Hafner et al. 2009; Álvarez-Castañeda 2024).
Distribution. Pappogeomys bulleri is known from the mountains, tablelands, and coastal plains near the western end of the Trans-Mexican Volcanic Belt in west-central México, including the states of Nayarit, Jalisco, and Colima (Hafner et al. 2009).
Comments. Cratogeomys was first considered a subgenus of Pappogeomys (Nelson and Goldman 1934; Russell 1968a); it is currently a full genus (Honeycutt and Williams 1982; Demastes et al. 2002). Hafner et al. (2009) reviewed the Pappogeomys bulleri complex (considering that the previous subspecies of P. bulleri are junior synonyms of the following subspecies: P. b. albinasus includes P. b. infuscus and P. b. nayaritensis (part of the species); P. b. bulleri includes P. b. amecensis, P. b. flammeus, P. b. lagunensis, and P. b. lutulentus; and P. b. burti includes P. b. melanurus. Cratogeomys differs from all the other species of the family Geomyidae in the following characteristics: rostrum wider than the interorbital region; without a zygomatic anterior angle with a plate-shaped expansion, and third upper molars not clearly showing two lobes.
Pappogeomys bulleri (Thomas, 1892)
Buller’s pocket gopher, tuza de Jalisco
1892. Geomys bulleri Thomas, Ann. Mag. Nat. Hist., ser. 6, 10:196. Type locality “near Talpa, W slope Sierra de Mascola, 8500 (probably about 5000) ft., Jalisco”.
1895. Pappogeomys bulleri, Merriam, N. Amer. Fauna, 8:159.
1. P. b. albinasus Merriam, 1895. Type locality “Atemajac, a suburb of Guadalajara, Jalisco”. Known only from central Jalisco and southeastern Nayarit.
2. P. b. alcorni Russell, 1957. Type locality “4 mi. W Mazamitla, 6600 ft., Jalisco”. Range in eastern and central Jalisco.
3. P. b. bulleri (Thomas, 1892). For type locality see above. Known only from the Sierra Madre del Sur highlands, Jalisco.
4. P. b. burti Goldman, 1939. Type locality “Tenacatita Bay, southwestern coast of Jalisco”. Known only from coastal areas and lowlands of Colima and Jalisco.
5. P. b. nayaritensis Goldman, 1939. Type locality “about 10 mi, S Tepic, 5000 ft., Nayarit”. Known only from lowlands of Nayarit.
Zygogeomys Merriam, 1895
1895. Zygogeomys Merriam, N. Amer. Fauna, 8:195. Type species Zygogeomys trichopus Merriam, 1895.
Content. One species of Zygogeomys is recognized: Zygogeomys trichopus Merriam, 1895.
Etymology. The name Zygogeomys is derived from the Greek zygos, meaning “zygomatic”; geo, "earth"; and mys, “mouse”: related to the shape of the zygomatic arch, which is characteristic of the genus (Merriam 1895).
Diagnosis. Body compact and cylindrical; eyes, ears, and limbs small; fore- and hindfoot claws well-developed; tail short and slightly darker dorsally; upper incisors bisulcate, a major sulcus on the inner side of the median line and a minor sulcus on the inner convexity; third upper molars conspicuously bicolumnar, longer than wide owing to the elongation of the posterior loph; rostrum narrow relative to its length; maxillary and squamosal roots of the zygomatic arches in contact above the jugal, and antero-extemal angles rounded rather than expanded; zygomata not widely spreading and slender; sagittal crest short but well-developed (Merriam 1895, Russell 1968a, Álvarez-Castañeda 2024).
Distribution. Zygogeomys is known only from Nahuatzen, Pátzcuaro, Cerros Tancítaro, and Patambán, all in the state of Michoacán, Mexico (Merriam 1895, Russell 1968a; Hall 1981).
Comments. Zygogeomys trichopus can be found in sympatry with Cratogeomys fumosus, which is normally more common and abundant. Zygogeomys trichopus can be distinguished from Cratogeomys by having two grooves in the anterior surface of the upper incisors (the internal grooves are very notorious, can be detected by passing a pencil tip or a thumb nail).
Zygogeomys can be differentiated from all other genera mainly by having the upper incisors bisulcate, a major sulcus on the inner side of the median line and a minor sulcus on the inner convexity; third upper molars conspicuously bicolumnar, longer than wide owing to the elongation of the posterior loph and maxillary and squamosal roots of the zygomatic arches in contact above the jugalclearly morphologicall distinct from other members of the sub-clade (Merriam 1895, Russell 1968a, Álvarez-Castañeda 2024).
Zygogeomys trichopus Merriam, 1895
Michoacán pocket gopher, tuza de Michoacán
1895. Zygogeomys trichopus Merriam, N. Amer. Fauna, 8:196. Type locality “Nahuatzen, Michoacán”.
1. Z. t. tarascensis Goldman, 1938. Type locality “6 mi. SE Patzcuaro, 8000 ft., Michoacán”. Known only from Pátzcuaro area, Michoacán.
2. Z. t. trichopus Merriam, 1895. For type locality see above. Known only from Nahuatzen, Cerros Tancítaro, and Patambán, Michoacán.
Sub-clade B2
Genus Heterogeomys Merriam, 1895
1895. Heterogeomys Merriam, N. Amer. Fauna, 8:179. Type species. Geomys hispidus Le Conte, 1852.
Content. Two species of the subgenus Heterogeomys are recognized: Heterogeomys (Heterogeomys) hispidus (Le Conte, 1852) and Heterogeomys (Heterogeomys) lanius Elliot, 1905, and five of the subgenus Macrogeomys are recognized: Heterogeomys (Macrogeomys) cavator (Bangs, 1902), Heterogeomys (Macrogeomys) cherriei (J. A. Allen, 1893), Heterogeomys (Macrogeomys) dariensis (Goldman, 1912), Heterogeomys (Macrogeomys) heterodus (Peters, 1865), and Heterogeomys (Macrogeomys) underwoodi (Osgood, 1931).
Etymology. The name Heterogeomys is derived from the Greek Hetero, meaning “other, different”; geo, "earth"; and mys, “mouse”: the other earth mouse or the different earth mouse (Jaeger 1955).
Diagnosis. The genus Heterogeomys comprises large specimens; coloration dark brown; pelage harsh and stiff; frontal bone narrow and not markedly inflated; interorbital region decidedly constricted; zygomata more widely spreading; postorbital bar (process) weakly developed; anterior margin of the mesoptergoid fossa even with the plane of the posterior wall of third upper molars; first upper incisors unisulcate; surface of incisors flat on both sides of the sulcus; sulcus wholly on the inner side of the median line and deep and abrupt on the inner third in some specimens; anteroposterior occlusal length of the third upper molars equal to or shorter than the combined lengths of the first and second upper molars; fourth upper premolars with a short enamel plate and restricted to the lingual end of the wall (Russell 1968a; Hall 1981).
Comments. Heterogeomys was first described as a full genus (Merriam 1895) and was later considered a subgenus of Orthogeomys (Russell 1968a). Molecular data supports its reassignment at the genus level, including Macrogeomys as a subgenus (Spradling et al. 2016).
Subenus Heterogeomys Merriam, 1895
Heterogeomys (Heterogeomys) hispidus (Le Conte, 1852)
Hispid pocket gopher, tuza gigante tropical
1852. G[eomys]. hispidus Le Conte, Proc. Acad. Nat. Sci. Philadelphia, 6:158. Type locality “near Jalapa, Veracruz”.
2016. Heterogeomys hispidus Spradling, Demastes, Hafner, Milbach, Cervantes, and Hafner, Jour. Mamm. 97:415
1. H. h. cayoensis Burt, 1937. Type locality “Mountain Pine Ridge, 12 mi. S El Cayo, British Honduras [Belize].”
2. H. h. chiapensis Nelson and Goldman, 1929. Type locality “Tenejapa, 16 mi. NE San Cristobal, Chiapas”. Range in all Chiapas, except northwestern.
3. H. h. concavus Nelson and Goldman, 1929. Type locality “Pinal de Amoles, Queretaro”. Range in San Luis Potosí, Querétaro, and northwestern Veracruz.
4. H. h. hispidus (Le Conte, 1852). For type locality see above. Range in western Veracruz and eastern Puebla.
5. H. h. hondurensis Davis 1966. Type locality “8 mi. W Tela, Honduras”. Known only from type locality.
6. H. h. isthmicus Nelson and Goldman, 1929. Type locality “Jaltipan, Veracruz”. Known only from southeastern Veracruz.
7. H. h. latirostris Hall and Álvarez, 1961. Type locality “Hda. Tamiahua, Cobo Rojo, Veracruz”. Known only from northeastern Veracruz.
8. H. h. negatus Goodwin, 1953. Type locality “Gómez Feras [= Farías], 1300 ft., about 45 mi. S Ciudad Victoria, 10 mi. W Pan American Highway, Tamaulipas”. Known only from southern Tamaulipas.
9. H. h. teapensis Goldman, 1939. Type locality “Teapa, Tabasco. Known only from type locality”. Known only from southern Tabasco and northwestern Chiapas.
10. H. h. tehuantepecus Goldman, 1939. Type locality “mountains 12 mi. NW Santo Domingo and about 60 mi. N Tehuantepec, 1600 ft., Oaxaca”. Known only from northern Oaxaca.
11. H. h. torridus Merriam, 1895. Type locality “Chichicaxtle, Veracruz”. Known only from central Veracruz.
12. H. h. yucatanensis Nelson and Goldman, 1929. Type locality “Campeche, Campeche”. Range in Yucatán peninsula, Belize, and Guatemala.
Heterogeomys (Heterogeomys) lanius Elliot, 1905
Big pocket gopher, tuza gigante de Veracruz
1905. Heterogeomys lanius Elliot, Proc. Biol. Soc. Washington, 18:235. Type locality “Xuchil, Veracruz”. Known only from type locality.
Subgenus Macrogeomys (Peters, 1895)
Heterogeomys (Macrogeomys) cavator (Bangs, 1902)
Chiriquí Pocket Gopher
1902. Macrogeomys cavator Bangs, Bull. Mus. Comp. Zool., 39:42. Type locality “Boquete [Chiriqui Province, Panama] 4,000 to 7,000 feet”.
2016. Heterogeomys cavator Spradling, Demastes, Hafner, Milbach, Cervantes, and Hafner, Jour. Mamm. 97:416.
1. H. c. cavator (Bangs, 1902). For type locality see above. Known only from Chiriqui Province, Panama.
2. H. c. nigrescens (Goodwin, 1934). Type locality “El Muñeco (Rio Navarro), 10 miles south of Cartago, Province Cartago, Costa Rica, altitude 4,000 feet”. Known only from type locality.
3. H. c. pansa (Bangs, 1902). Type locality “Bogaba [Bugaba], Chiriqui Province, Panama”. Known only from type locality.
Heterogeomys (Macrogeomys) cherriei (J. A. Allen, 1893)
Cherrie’s Pocket Gopher
1893. Geomys cherriei J. A. Allen, Bull. Amer. Mus. Nat. Hist., 5:337. Type locality “Santa Clara, Costa Rica.
2016. Heterogeomys cherriei Spradling, Demastes, Hafner, Milbach, Cervantes, and Hafner, Jour. Mamm. 97:416.
1. H. c. carlosensis (Goodwin, 1934). Type locality “Cataratos, San Carlos, Province Alajuela, Costa Rica, about 400 feet elevation”. Known only from Province Alajuela, Costa Rica.
2. H. c. cherriei (J. A. Allen, 1893). For type locality see above. Known only from Santa Clara area, Costa Rica.
3. H. c. costaricensis (Merriam, 1895). Type locality “Pacuare, Costa Rica”. Known only from Pacuare Basin, Costa Rica.
4. H. c. matagalpae (J. A. Allen, 1910). Type locality “Pena [Peña] Blanca, Matagalpa, Nicaragua”. Range in central area of Nicaragua.
Heterogeomys (Macrogeomys) dariensis (Goldman, 1912)
Darién Pocket Gopher
1912. Macrogeomys dariensis Goldman, Smiths. Miscl. Coll., 60(2):8. Type locality “Cana (altitude 2,000 feet) in the mountains of eastern Panama”.
2016. Heterogeomys dariensis Spradling, Demastes, Hafner, Milbach, Cervantes, and Hafner, Jour. Mamm. 97:416.
1. H. d. dariensis (Goldman, 1912). For type locality see above. Known only from The Darien, southern Panama.
2. H. d. thaeleri (Alberico, 1990). Type locality “ca. 7 km S Bahía Solano, Municipio Bahía Solano, Departamento del Chocó, Colombia, ca. 100 m”. Known only from Municipio Bahía Solano northwestern Colombia.
Heterogeomys (Macrogeomys) heterodus (Peters, 1865)
Variable Pocket Gopher
1865. Geomys heterodus Peters, Monatsb. preuss. Akad. Wiss., Berlin, p. 177). Type locality “Costa Rica”.
2016. Heterogeomys heterodus Spradling, Demastes, Hafner, Milbach, Cervantes, and Hafner, Jour. Mamm. 97:417.
1. H. h. cartagoensis (Goodwin, 1934). Type locality “Paso Ancho, Province Cartago, Costa Rica”. Central area of Costa Rica.
2. H. h. dolichocephalus (Merriam, 1895). Type locality “San Jose, Costa Rica”. Known only from the area of San José, Costa Rica.
3. H. h. heterodus (Peters, 1865). For type locality see above. Known only from the area of Escazú, Costa Rica.
Heterogeomys (Macrogeomys) underwoodi (Osgood, 1931)
Underwood’s Pocket Gopher
1931. Macrogeomys underwoodi Osgood, Field Mus. Nat. Hist., Publ. 295, Zool. Ser., 18(5):143. Type locality “Alto de Jabillo Pirris, between San Geronimo and Pozo Azul, western Costa Rica”.
2016. Heterogeomys underwoodi Spradling, Demastes, Hafner, Milbach, Cervantes, and Hafner, Jour. Mamm. 97:417.
Sub-clade B3
Genus Geomys Rafinesque, 1817
1817. Geomys Rafinesque, Amer. Month. Mag., 2:45. Type Geomys pinetis Rafinesque, 1817.
Content. Thirteen allopatric species are recognized species of Geomys are recognized: Geomys arenarius Merriam, 1895, Geomys attwateri Merriam, 1895, Geomys breviceps Baird, 1855, Geomys bursarius (Shaw, 1800), Geomys jugossicularis Hooper, 1940, Geomys knoxjonesi Baker and Genoways, 1975, Geomys lutescens Merriam, 1890, Geomys mobilensis (Merriam, 1895), Geomys personatus True, 1889, Geomys pinetis Rafinesque, 1817, Geomys streckeri Davis, 1943, Geomys texensis Merriam, 1895, Geomys tropicalis Goldman, 1915.
Etymology. The name Geomys is derived from the Greek geo, "earth", and mys, “mouse”: earth mouse (Álvarez-Castañeda and Álvarez 1996).
Diagnosis. Dorsal pelage reddish to grayish brown; fur of underparts paler than in the dorsum; back of the rostrum and head darker, contrasting with the nape and back; rump generally paler; whitish gray coloration from chin to neck; body compact and cylindrical; eyes, ears, and limbs small; claws of limbs well-developed; skull large and massive; ridge on the squamosal joining the temporal in adult and old males; interorbital region narrower than the rostrum; middle part of anterior surface of upper incisors with two grooves: one large, deep, and medial; the other small and flanked on the inner side; first upper molars without an enamel plate and larger than the first lower molars; upper molariforms not prominently bicolumnar and almost as long as wide; all molariforms elliptical with a small anterior-posterior axis; anterior and posterior margins of molariforms with enamel, other margins with dentin; sagittal crest poorly developed (Russell 1968a; Baker and Williams 1974; Sudman et al. 2006; Álvarez-Castañeda 2024).
Distribution. Geomys ranges east of the Mississippi River in Alabama, Georgia, and Florida, associated with deep sandy soils and open areas in long-leaf pinewood forests, and west of the Mississippi River from southern Manitoba, Canada, southward throughout northeastern Tamaulipas, Mexico, and westward throughout eastern Wyoming, Colorado, central New Mexico, western Texas, and northern Chihuahua.
Comments. G. p. colonus, G. p. cumberlandius, and G. p. floridanus were recognized as distinct species (Hall 1981; Laerm 1981), but not by Patton (1993, 2005) and Baker et al. (2003). The subspecies G. p. austrinus, G. p. colonus, G. p. cumberlandius, G. p. floridanus, G. p. goffi, G. p. mobliensis are considered junior synonyms of G. p. pinetis (Williams and Genoway 1980). Sudman et al. (2006) considered that G. p. mobilensis may represent a species different from G. pinetis. No other species of the genus Geomys occurs anywhere near its range. G. pinetis cannot be differentiated morphologically from any other species west to the Mississippi River. The chromosome number is 2n = 42, FN = 80.
Geomys shows a high genetic difference (17.6 % [16.1 %–19.0 %]) relative to all other species east of the Mississippi River, higher than the difference within species within any genus of the family Geomyidae and the species east of the Mississippi River 12.4 % (2.8 %–15.3 %). Additiionally, Geomys compared to all other species of eastern of the Missiissippi River have presence-absence of lice species (Hafner et al. 1994), protein (Kennedy 1988), mtDNA restriction patterns, and allozyme loci (Avise et al. 1979), with the Mississippi River acting as an impassable barrier that isolated Geomys from all other species of eastern of the Mississippi River.
Geomys shows a high inner variation, so its analysis considered two geographical groups in relation to the Mississippi River determined based on the following conditions: 1) presence of different species complexes determined by several authors (Russell 1968a; Williams and Genoway 1980; Hall 1981; Sudman et al. 2006); 2) the marked difference in the nasal shape, being straight in western specimens vs nasals with a strong constriction near the middle in eastern specimens (hourglass-shaped); interorbital area narrow (western) vs a notorious narrow interorbital area (eastern); the east-of-Mississippi population is a different clade from the population thriving in the west side, and is considered basal (Russell 1968a; Penney and Zimmerman 1976; Sudman et al. 2006). The Mississippi River is a mayor barrier for the gopher dispersion, flows from northern Minnesota to the Gulf of Mexico, dividing inland plains by a large stream that is virtually impossible to cross by gophers. We consider the crossing of the river as a hypothesis, which a population was located near the boundary of a meander belt and dispersed into the neck of one lobe of the river that later was cut-off from the mainstream, the population could have crossed the river and diversified. The Mississippi River was established as a geographic barrier that prevented subsequent dispersion events.
Geomys arenarius Merriam, 1895
Desert pocket gopher, tuza del desierto
1895. Geomys arenarius Merriam, N. Amer. Fauna, 8:139. Type locality “El Paso Co., Texas”.
1. G. a. arenarius Merriam, 1895. For type locality see above. Range in southern New Mexico, northern Chihuahua, and southwestern Texas.
2. G. a. brevirostris Hall, 1932. Type locality “E edge [white] sand [9 mi. W Tularosa], Tularosa-Hot Springs Road, Otero Co., New Mexico”. Known only from central-southern New Mexico.
Geomys attwateri Merriam, 1895
Attwater´s pocket gopher, tuza de Corpus Christi
1895. Geomys breviceps attwateri Merriam, N. Amer. Fauna, 8:135. Type locality “Rockport, Texas”.
1. G. a. ammophilus Davis, 1940. Type locality “Cuero, De Witt Co., Texas”. Known only from the Brazos River Basin near the coast, central-southeastern Texas.
2. G. a. attwateri Merriam, 1895. For type locality see above”. Known only from the Colorado and Guadalupe River basins near the coast of southeastern Texas.
Geomys breviceps Baird, 1855
Baird´s pocket gopher, tuza texana del este
1855. Geomys breviceps Baird, Proc. Acad. Nat. Sci. Philadelphia, 7:335. Type locality “Prairie Mer Rouge, Lousiana. Known only from Morehouse Parish”.
1. G. b. breviceps Baird, 1855. For type locality see above. Range from southeastern Oklahoma and southwestern Arkansas south through eastern Texas and western Louisiana.
2. G. b. ozarkensis Elrod, Zimmerman, Sudman, and Heidt, 2000. Type locality “from 3 mi S Melbourne, Izard County, Arkansas”. Range in northern-central Arkansas.
3. G. b. sagittalis Merriam, 1895. Type locality “Clear Creek, Galveston Bay, Galveston Co., Texas”. Known only from Galveston Bay, Texas.
Geomys bursarius (Shaw, 1800)
Plains pocket gopher, tuza de las planicies
1800. Mus bursarius Shaw, Trans. Linnean Soc. London, 5:227. Type locality “somewhere in upper Mississippi Valley (Restricted to Elk River, Sherburne Co., Minnesota, by Swenk, Missouri Valley Fauna, 1:6.)”.
1829. Geomys bursarius, Richardson, Fauna BorealiAmericana 1:203.
1. G. b. bursarius (Shaw, 1800). For type locality see above. Range from southern Manitoba south through eastern Dakotas, Minnesota, and northwestern Wisconsin.
2. G. b. illinoensis Komarek and Spencer, 1931. Type locality “1 mi, S Momence, Kankakee Co., Illinois”. Range in central Illinois and central-western Indiana.
3. G. b. majusculus Swenk, 1939. Type locality “Lincoln, Nebraska”. Range from Iowa and northern-central Missouri west through southeastern Nebraska and eastern Kansas.
4. G. b. missouriensis McLaughlin, 1958. Type locality “2 mi. N Manchester, St. Louis Co., Missouri”. Range in eastern Missouri.
5. G. b. wisconsinensis Jackson, 1957. Type locality “Lone Rock, Richland Co., Wisconsin”. Range in western Wisconsin.
Geomys jugossicularis Hooper, 1940
Hall´s pocket gopher, tuza de las planicies centrales
1940. Geomys lutescens jugossicularis Hooper, Occas. Pap. Mus. Zool., Univ. Michigan, 420:1. Type locality “Lamar, Prowers Co., Colorado”.
1. G. j. halli Sudman, Choates and Zimmerman, 1987. 1 3/4 mi. E Ellis (Tl3S, R20W, NE 1/4 Sec. 10), Ellis Co., Kansas. Range from eastern Colorado east through southwestern Kansas, Oklahoma, and northeastern New Mexico.
2. G. j. jugossicularis Hooper, 1940. For type locality see above. Oklahoma and southwestern Kansas.
Geomys knoxjonesi Baker and Genoways, 1975
Jones´s pocket gopher, tuza texana del oeste
1975. Geomys bursarius knoxjonesi Baker and Genoways, Occas. Pap. Mus. Texas Tech Univ., 29:1. Type locality “4.1 mi. N, 5.1 mi. E Kermit, Winkler Co., Texas”.
Geomys lutescens Merriam, 1890
Sandy hill pocket gopher, tuza arenera
1890. Geomys bursarius lutescens Merriam, N. Amer. Fauna, 4:51. Type locality “sandhills on Birdwood Creek, Lincoln Co., Nebraska”.
1. G. l. industrius Villa and Hall, 1947. Type locality “1 1/2 mi. N Fowler, Meade Co., Kansas”. Known only from southwestern Kansas.
2. G. l. lutescens Merriam, 1890. For type locality see above. Range from South Dakota south through central-eastern Colorado and central Kansas.
3. G. l. major Davis, 1940. Type locality “8 mi. W Clarendon, Donley Co., Texas”. Range from southern Kansas south through.
Geomys personatus True, 1889
Texas pocket gopher, tuza texana del sur
1889. Geomys personatus True, Proc. U.S. Nat. Mus., 11:159 for 1888. Type locality “Padre Island, Cameron Co., Texas”.
1. G. p. davisi Williams and Genoways, 1981. Type locality “3 mi N, 2.8 mi W Zapata, Zapata Co., Texas”. Known only from southern Texas.
2. G. p. fallax Merriam, 1895. Type locality “S side Nueces Bay, Nueces Co. Texas”. Known only from southeastern Texas.
3. G. p. fuscus Davis, 1940. Type locality “Fort Clark [Bracketville], Kinney Co., Texas”. Known only from Kinney and Valverde counties, southern Texas.
4. G. p. maritimus Davis, 1940. Type locality “Flour Bluff, 11 mi. SE Corpus Christi, Nueces Co., Texas”. Known only from Baffin Bay and Flour Bluff, southern Texas.
5. G. p. megapotamus Davis, 1940. Type locality “4 mi. SE Oilton, Webb Co., Texas”. Range in southern Texas and northeastern Tamaulipas.
6. G. p. personatus True, 1889. For type locality see above. Known only from the Mustang and Padre islands, southern Texas.
Geomys pinetis Rafinesque, 1817
Southeastern pocket gopher, tuza del sureste
1806. Mus tuza Barton, Mag. fur den neuesten Zustand der Naturkunde (ed. J. H. Voight), 12(6):488 (Type locality restricted to pine barrens near Augusta, Georgia, by Bangs, Proc. Boston Soc. Nat. Hist., 28:175. According to Harper, Proc. Biol. Soc. Washington, 65:36, 952, tuza of Barton is of uncertain application and is regarded as not available.)
1817. Geomys pinetis Rafinesque, Amer. Month. Mag., 2(1):45. Type locality “Georgia in the region of the pines (More restrictedly, Screven County according to Harper, Proc. Biol. Soc. Washington, 65:36, 1952.)” Regarded as identical with tuza by Merriam, N. Amer. Fauna, 8:113, January 31, 1895.
1. G. p. fontanelus (Sherman, 1940). Type locality “7 mi. NW Savannah, Chatham Co., Georgia”. Known only from Chatham County, eastern Georgia.
2. G. p. pinetis (Rafinesque, 1817). For type locality see above. Range in Georgia and Florida.
Geomys mobilensis Merriam, 1895
Southeastern Pocket Gopher of Mobile Bay, tuza del sureste de la bahía de Mobile
1895. Geomys tuza mobilensis Merriam, N. Amer. Fauna, 8:119. Type locality “Point Clear, Mobile Bay, Baldwin Co., Alabama”.
2006. Geomys mobilensis: Sudman, Wickliffe, Horner, Smolen, Bickham, and Bradley, J. Mamm. 87:674.
Geomys streckeri Davis, 1943
Strecker´s pocket gopher, tuza texana del Carrizo
1940. Geomys personatus minor Davis, Texas Agric. Exp. Station Bull., 590:29. Type locality “Carrizo Springs, Dimmit Co., Texas”. Not Geomys minor Gidley, 1922, a fossil. Known only from type locality.
Geomys texensis Merriam, 1895
Llano pocket gopher, tuza del centro de Texas
1895. Geomys texensis Merriam, N. Amer. Fauna, 8:137. Type locality “Mason, Mason Co., Texas.
1. G. t. bakeri Smolen, Pitts and Bickham, 1993. Type locality “1 mile E D'Hanis, Medina Co., Texas”. Known only from Medina, Uvalde, and Zavala counties, central-south Texas.
2. G. t. llanensis Bailey, 1905. Type locality “Llano, Texas”. Known only from Gillespie, Kimble, and Zavala counties, central-southern Texas.
3. G. t. texensis Merriam, 1895. For type locality see above. Known only from Mason, McCulloch, and San Saba counties, central Texas.
Geomys tropicalis Goldman, 1915
Tropical pocket gopher, tuza tropical
1915. Geomys personatus tropicalis Goldman, Proc. Biol. Soc. Washington, 28:134. Type locality “Altamira, Tamaulipas”.
Supplementary material
Supplementary material 1. GenBank accession numbers for the cytochrome b and cytochrome oxidase subunit 1 sequences and the authors who recorded the sequences in GenBank used in the present study.
https://mastozoologiamexicana.com/therya/index.php/THERYA/article/view/6153/1468
Supplementary material 2. Phylogenetic inference using neighbor-joining for the cytochrome b sequences using the large set of data.
https://mastozoologiamexicana.com/therya/index.php/THERYA/article/view/6153/1469
Supplementary material 3. Phylogenetic inference using neighbor-joining, unweighted pair group method with arithmetic mean (UPGMA), maximum-parsimony, maximum-likelihood, and Bayesian inference for the cytochrome b and cytochrome oxidase subunit 1 sequences.
https://mastozoologiamexicana.com/therya/index.php/THERYA/article/view/6153/1470
Supplementary material 4. Percentage of genetic differences of Cytb between the species examined in the family Geomyidae.
https://mastozoologiamexicana.com/therya/index.php/THERYA/article/view/6153/1471

Figure 1. Phylogenetic analysis based on 46 specimens (1,141 bp) using cytochrome b gene sequences. The sequences represent individuals of different species belonging to the family Geomyidae. This tree supports the monophyly of ten clades within the Geomyidae. Each clade represents one genus.
Table 1. Pairwise percentage of genetic differences based on cytochrome b (Cytb) across genera and subgenera in the family Geomyidae.
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
||
|
1 |
Cratogeomys |
||||||||||
|
2 |
Pappogeomys |
15.70% |
|||||||||
|
14.04-16.77 |
|||||||||||
|
3 |
Zygogeomys |
15.99% |
15.50% |
||||||||
|
13.74-18.42 |
14.73-15.95 |
||||||||||
|
4 |
Orthogeomys |
15.97% |
13.03% |
15.94% |
|||||||
|
13.27-18.42 |
6.76-15.48 |
15.24-16.23 |
|||||||||
|
5 |
Heterogeomys |
16.47% |
16.51% |
16.45% |
14.81% |
||||||
|
(subgenus) |
13.10-18.70 |
14.86-18.19 |
15.87-17.11 |
2.64-17.67 |
|||||||
|
6 |
Macrogeomys |
16.36% |
16.54% |
15.38% |
15.36% |
11.09% |
|||||
|
(subgenus) |
14.65-17.72 |
15.96-17.01 |
14.88-15.79 |
14.43-16.32 |
9.11-11.67 |
||||||
|
7 |
Geomys |
17.95% |
16.34% |
16.65% |
16.66% |
18.23% |
17.09% |
||||
|
(west Mississippi) |
16.23-20.32 |
15.28-17.37 |
14.56-18.49 |
12.65-19.34 |
16.27-19.74 |
15.92-18.21 |
|||||
|
8 |
Geomys |
17.55% |
16.56% |
16.31% |
14.94% |
17.62% |
16.85% |
16.76% |
|||
|
(east Mississippi) |
16.32-18.74 |
16.36-16.83 |
16.19-16.41 |
12.74-16.80 |
15.96-18.60 |
16.23-18.07 |
15.48-17.83 |
||||
|
9 |
Megascapheus |
20.71% |
19.85% |
20.27% |
21.64% |
20.59% |
18.96% |
20.15% |
19.72% |
||
|
(subgenus) |
18.77-22.98 |
18.49-21.71 |
18.74-22.15 |
18.95-25.40 |
17.60-22.09 |
17.47-21.48 |
17.98-22.65 |
17.33-21.75 |
|||
|
10 |
Thomomys |
20.74% |
21.15% |
19.61% |
21.63% |
20.31% |
19.18% |
20.91% |
20.02% |
19.92% |
|
|
(subgenus) |
19.47-21.99 |
20.59-21.75 |
18.78-21.40 |
20.44-23.89 |
17.52-22.55 |
16.97-21.23 |
19.65-22.46 |
19.38-20.53 |
18.16-21.23 |
||
|
0 |
outgroup |
23.31% |
22.92% |
22.80% |
23.82% |
22.61% |
21.66% |
23.15% |
23.01% |
23.36% |
24.01% |
|
20.88-25.50 |
21.23-25.73 |
20.58-24.73 |
21.30-27.79 |
19.35-24.50 |
19.48-24.06 |
11.34-25.81 |
11.34-25.81 |
21.63-25.81 |
22.72-25.36 |
Table 2. Pairwise percentage of genetic differences based on cytochrome oxidase subunit 1 (COI) across genera and subgenera in the family Geomyidae.
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
||
|
1 |
Cratogeomys |
||||||||||
|
2 |
Pappogeomys |
13.99% |
|||||||||
|
12.95-15.14 |
|||||||||||
|
3 |
Zygogeomys |
14.73% |
14.46% |
||||||||
|
13.65-16.02 |
14.27-14.64 |
||||||||||
|
4 |
Orthogeomys |
14.59% |
15.51% |
14.56% |
|||||||
|
13.71-16.10 |
15.35-15.67 |
14.04-15.09 |
|||||||||
|
5 |
Heterogeomys |
15.09% |
13.56% |
16.05% |
14.46% |
||||||
|
(subgenus) |
14.25-16.34 |
12.82-14.18 |
15.55-16.37 |
14.05-14.89 |
|||||||
|
6 |
Macrogeomys |
15.98% |
14.78% |
15.18% |
15.24% |
10.44% |
|||||
|
(subgenus) |
15.04-17.10 |
14.18-15.59 |
14.21-16.12 |
13.99-16.06 |
10.04-10.96 |
||||||
|
7 |
Geomys |
16.23% |
15.69% |
15.89% |
15.72% |
17.41% |
17.41% |
||||
|
(west Mississippi) |
15.23-17.74 |
15.03-16.32 |
15.29-16.43 |
15.22-16.32 |
16.32-18.41 |
16.32-18.41 |
|||||
|
8 |
Geomys |
16.31% |
14.70% |
15.43% |
15.48% |
15.47% |
15.47% |
14.66% |
|||
|
(east Mississippi) |
15.29-16.71 |
14.31-15.09 |
15.35-15.51 |
15.41-15.54 |
14.96-15.87 |
14.96-15.87 |
13.67-15.93 |
||||
|
9 |
Megascapheus |
18.32% |
18.28% |
14.57% |
18.20% |
18.62% |
18.62% |
18.25% |
18.43% |
||
|
(subgenus) |
16.97-19.86 |
17.36-19.17 |
12.24-16.43 |
17.49-19.44 |
17.86-19.66 |
17.86-19.66 |
16.72-19.43 |
17.75-19.32 |
|||
|
10 |
Thomomys |
18.21% |
17.73% |
18.05% |
17.86% |
18.17% |
18.17% |
17.52% |
17.42% |
15.46% |
|
|
(subgenus) |
16.91-19.11 |
17.16-18.33 |
17.51-18.78 |
17.29-18.34 |
17.25-18.91 |
17.25-18.91 |
16.66-18.54 |
16.58-17.88 |
8.34-19.49 |
||
|
11 |
outgroup |
20.00% |
20.41% |
19.50% |
20.22% |
20.07% |
20.07% |
20.34% |
20.04% |
20.11% |
19.97% |
|
17.62-21.76 |
19.17-21.83 |
17.97-20.40 |
18.58-21.32 |
18.58-21.88 |
18.58-21.88 |
18.33-22.41 |
18.85-21.31 |
18.13-21.78 |
17.80-22.41 |
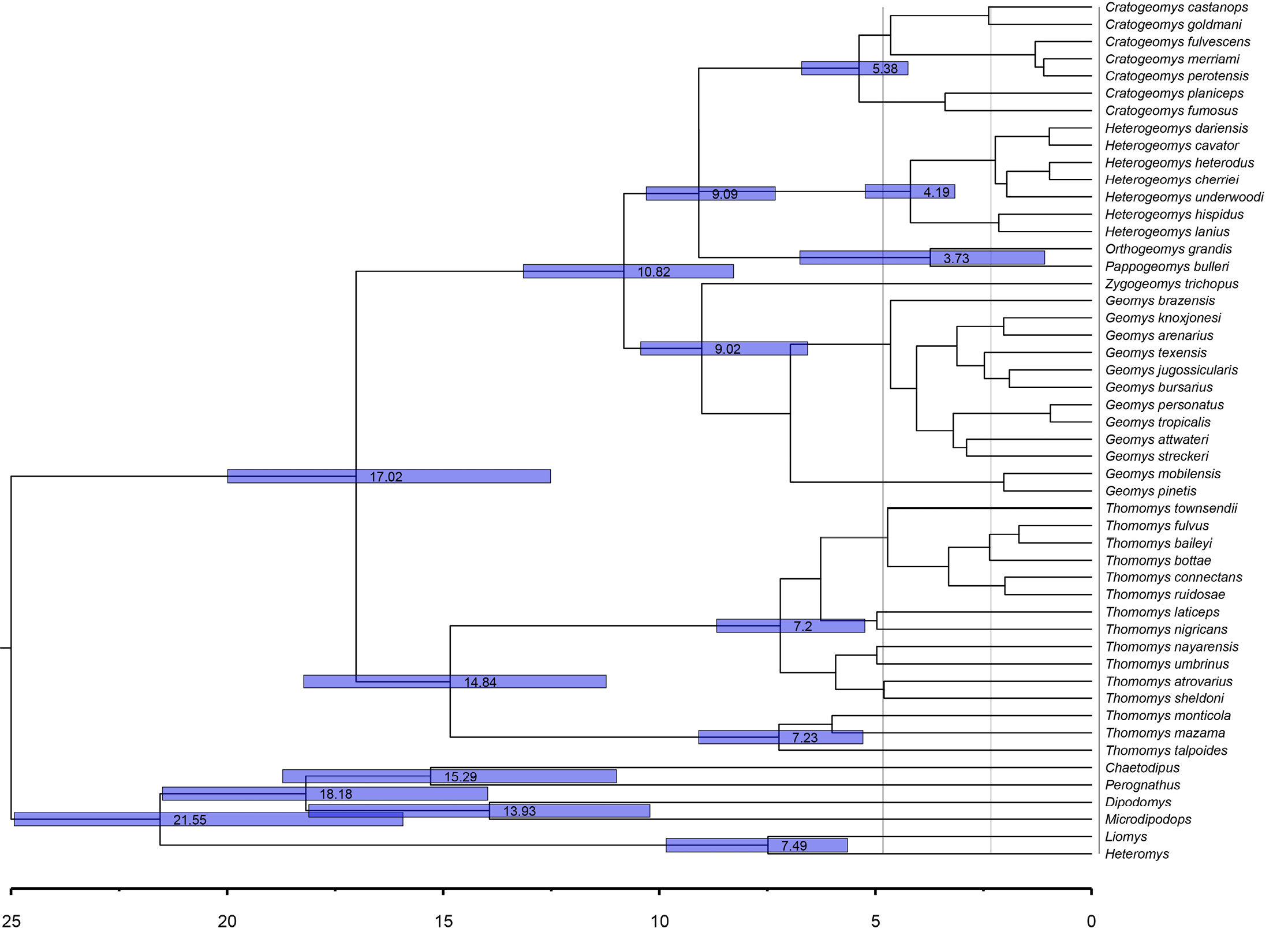
Figure 2. Calibrated maximum clade credibility tree of the Geomyidae and Heteromyidae using Cytb. Node labels include divergence time estimates in millions of years. Horizontal bars show the 95 % highest posterior density intervals surrounding each estimate. Calibration points were obtained from a Bayesian divergence dating analysis for the Heteromyidae using multigenes data (Hafner et al. 2007).
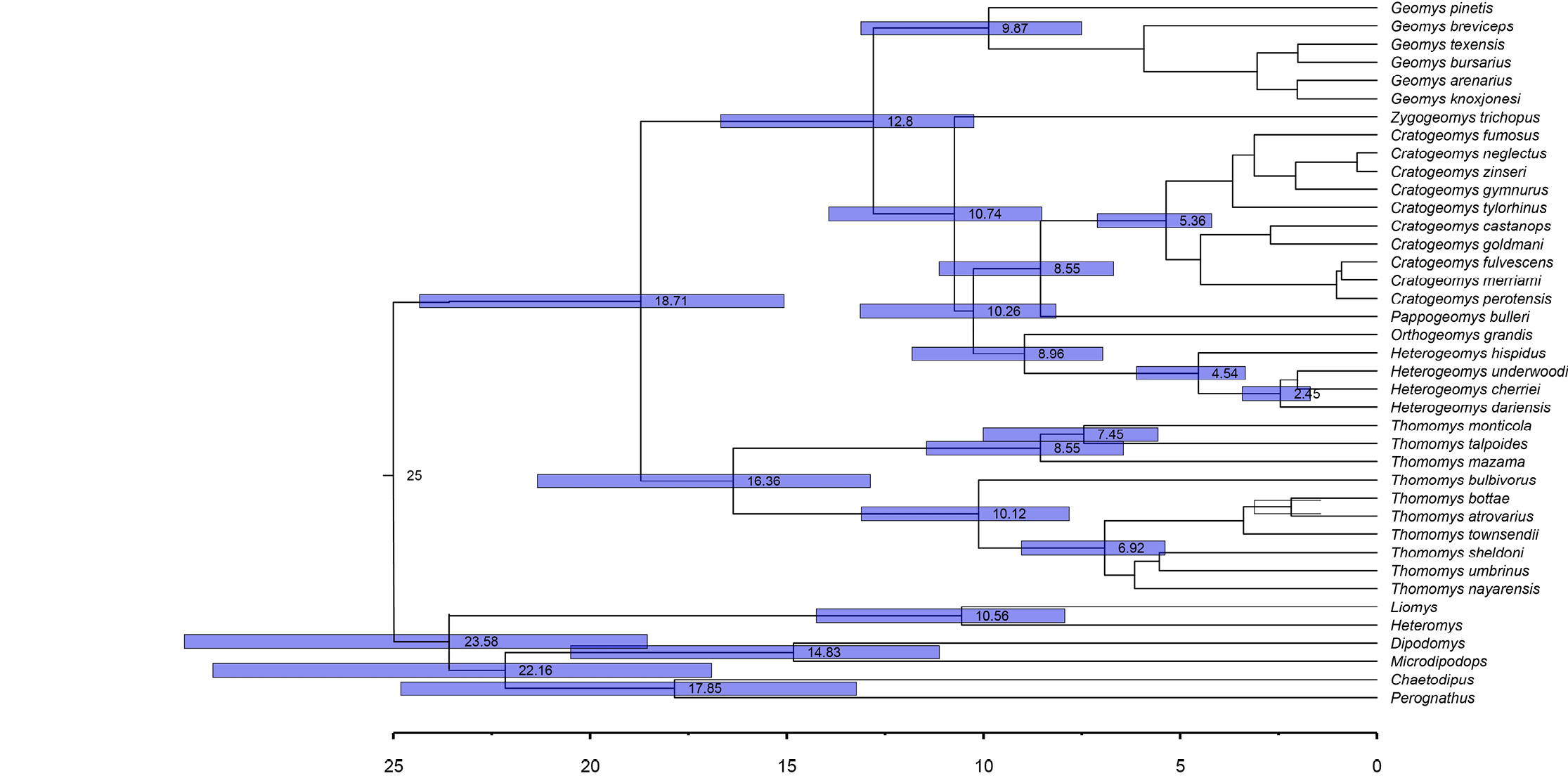
Figure 3. Calibrated maximum clade credibility tree of the Geomyidae and Heteromyidae using COI. Node labels include divergence time estimates in millions of years. Horizontal bars show the 95 % highest posterior density intervals surrounding each estimate. Calibration points were obtained from a Bayesian divergence dating analysis for the Heteromyidae using multigenes data (Hafner et al. 2007).
Table 3. Average and range of genetic differences based on Cytb and COI within each of the species examined in the family Geomyidae. *Only one individual was recorded.
|
Cytb |
COI |
|
|
Species |
Average (Min – Max) |
Average (Min – Max) |
|
Cratogeomys castanops |
1.42% (0.09 - 3.07) |
2.45% (0.00 - 4.07) |
|
Cratogeomys fulvescens |
0.39% (0.00 - 0.61) |
0.48% (0.13 - 0.78) |
|
Cratogeomys fumosus |
1.87% (1.14 - 2.46) |
* |
|
Cratogeomys goldmani |
2.75% (0.49 - 5.15) |
0.03% (0.01 - 0.04) |
|
Cratogeomys gymnurus |
2.81% (0.44 - 4.04) |
* |
|
Cratogeomys merriami |
2.58% (0.00 - 4.74) |
0.99% (0.00% 1.82) |
|
Cratogeomys neglectus |
0.72% (0.26 - 4.38) |
* |
|
Cratogeomys perotensis |
1.26% (0.00 - 2.02) |
0.93% (0.06 - 1.49) |
|
Cratogeomys planiceps |
0.94% (0.53 - 1.23) |
|
|
Geomys arenarius |
3.34% (0.00 - 6.24) |
* |
|
Geomys attwateri |
2.43% (1.32 - 3.84) |
|
|
Geomys bursarius |
3.55% (0.00 - 5.61) |
* |
|
Geomys jugossicularis |
1.83% (0.18 - 2.77) |
|
|
Geomys knoxjonesi |
2.12% (0.09 - 2.72) |
* |
|
Geomys personatus |
0.66% (0.00 - 0.96) |
|
|
Geomys mobilensis |
0.31% (0.00 - 0.56) |
|
|
Geomys pinetis |
2.02% (0.18 - 3.05) |
0.04% (0.00 - 0.06) |
|
Geomys streckeri |
0.43% (0.00 - 0.89) |
|
|
Geomys texensis |
1.44% (0.00 - 2.89) |
0.58% (0.58 - 0.58) |
|
Geomys tropicalis |
1.05% (0.52 - 1.57) |
|
|
Orthogeomys cavator |
2.89% (2.89 - 2.89) |
|
|
Orthogeomys cherriei |
0.72% (0.26 - 1.23) |
* |
|
Orthogeomys dariensis |
0.25% (0.00 - 0.53) |
* |
|
Orthogeomys grandis |
* |
3.98% (2.26 - 5.14) |
|
Orthogeomys heterodus |
0.24% (0.00 - 0.75) |
|
|
Orthogeomys hispidus |
0.39% (0.00 - 0.79) |
1.42% (0.34 - 2.74) |
|
Orthogeomys lanius |
* |
|
|
Orthogeomys underwoodi |
1.34% (0.76 - 1.76) |
* |
|
Pappogeomys bulleri |
5.83% (0.60 - 8.01) |
3.89% (0.00 - 5.26) |
|
Thomomys atrovarius |
4.59% (0.35 - 5.88) |
9.02% (2.01 - 4.88) |
|
Thomomys bottae |
3.14% (0.96 - 4.74) |
5.65% (0.00 - 4.50) |
|
Thomomys bulbivorus |
* |
|
|
Thomomys fulvus |
4.58% (0.20 - 6.83) |
|
|
Thomomys laticeps |
3.30% (2.02 - 4.24) |
|
|
Thomomys mazama |
0.58% (0.09 - 0.88) |
* |
|
Thomomys monticola |
0.06% (0.00 - 0.09) |
* |
|
Thomomys nayarensis |
0.61% (0.00 - 1.23) |
0.65% (0.65 - 0.65) |
|
Thomomys nigricans |
1.74% (0.20 - 2.61) |
|
|
Thomomys sheldoni |
4.47% (0.79 - 6.23) |
4.21% (3.44 - 5.39) |
|
Thomomys talpoides |
14.6% (6.32 - 16.84) |
5.27% (0.00%- 9.91) |
|
Thomomys townsendii |
9.62% (9.62 - 9.62) |
* |
|
Thomomys umbrinus |
3.89% (0.18 - 5.90) |
* |
|
Zygogeomys trichopus |
* |
* |
Table 4. Average and range of genetic differences based on Cytb and COI within each of the taxa examined in the family Geomyidae. *Only one species has been sequenced. ** Only two species have been sequenced.
|
Taxa |
Cytb |
COI |
|
Average (Min – Max) |
Average (Min – Max) |
|
|
Cratogeomys (genus) |
11.81% (4.64 - 15.08) |
9.45% (2.59 -11.96) |
|
Pappogeomys (genus) |
* |
* |
|
Zygogeomys (genus) |
* |
* |
|
Orthogeomys (genus) |
* |
* |
|
Heterogeomys (genus) |
10.62% (3.91 – 17.67) |
8.62% (6.84 - 10.96) |
|
Heterogeomys (subgenus) |
7.01%** |
1.09%** |
|
Macrogeomys (subgenus) |
7.92% (3.91 -11.31) |
7.50% (6.77 - 8.69) |
|
Geomys (genus) |
13.58% (3.24 – 19.03) |
11.76% (6.93 - 15.93) |
|
Geomys (W Mississippi) |
12.40% (4.90 – 19.03) |
10.05% (6.93 – 13.34) |
|
Geomys (E Mississippi) |
* |
* |
|
Thomomys (genus) |
16.09% (4.21 – 21.22) |
14.40% (6.28 - 19.49) |
|
Megascapheus (subgenus) |
14.01% (4.21 - 18.21) |
12.69% (6.29 - 15.61) |
|
Thomomys (subgenus) |
17.71% (7.01 – 20.08) |
14.46% (12.74 – 16.12) |