THERYA, 2025, Vol. 16(1):163-174 DOI:10.12933/therya-25-6174 ISSN 2007-3364
Riparian woodrat and black rat competition: investigating the role of an exotic species in the decline of a native endangered species
Brian L. Cypher1*, Patrick A. Kelly1, Karen L. Sproull1, Daniel F. Williams1, and Scott E. Phillips1
1 Endangered Species Recovery Program, California State University-Stanislaus, One University Circle, 95382, Turlock. California, USA. Email: bcypher@esrp.csustan.edu (BLC); pkelly@csustan.edu (PAK); biogirl999@gmail.com (KLS); danw1942@gmail.com (DFW); sphillips@esrp.csustan.edu (SEP).
* Corresponding author: https://orcid.org/0000-0002-7349-545X.
Competition from non-native species constitutes a significant threat to numerous native species worldwide. Endangered riparian woodrats (Neotoma fuscipes riparia) may be restricted to a single population occupying approximately 100 ha at Caswell Memorial State Park (CMSP) in central California. This population is vulnerable to a number of threats including from non-native black rats (Rattus rattus) that co-occur at CMSP. Black rats potentially engage in both interference and exploitative competition with woodrats. From September 2001 to September 2004, we investigated interactions between riparian woodrats and black rats to determine whether competitive interactions were reducing woodrat abundance or reproductive success. Two sites with riparian woodrats were identified at CMSP. Between January 2003 and September 2004, 179 black rats were removed from one site. Abundance of both species and woodrat reproductive success were assessed through live-trapping and radio-telemetry. Mean litter size, mean number of young for litters with emerged young, and mean number of young per female were all higher on the black rat removal site compared to the control site. Also, mean number of young for litters with emerged young increased on the removal site from 2003 to 2004 and decreased on the control site between years. Woodrat abundance trends were more equivocal and actually were higher on the control site during most of the period of black rat removal. The results of this investigation suggest that black rats may indeed suppress reproductive success of riparian woodrats, and that black rat removal could benefit woodrats. Black rats have been implicated in the declines and even extinction of other native rodents, including other woodrat species. Thus, black rats may constitute a significant threat to riparian woodrats, particularly in concert with other threats such as flooding, wildfires, and continued habitat loss and degradation. Therefore, we recommend that black rats be removed quarterly from highly suitable woodrat habitat in the CMSP. We also recommend that surveys be conducted to identify additional riparian woodrat populations and that black rat removals be conducted in those populations as well.
La competencia entre especies exoticas constituye una amenaza significativa para numerosas especies nativas en todo el mundo. Las ratas montera ribereñas (Neotoma fuscipes riparia) considerada en peligro de extinción pueden estar restringidas a una sola población que ocupa aproximadamente 100 ha en el Parque Estatal Caswell Memorial (CMSP) en el centro de California. Esta población es vulnerable a una serie de amenazas, incluidas las de ratas negras introducidas (Rattus rattus) que coexisten en CMSP. Las ratas negras potencialmente participan tanto en interferencia como en competencia de explotación con las ratas del bosque. Desde septiembre de 2001 hasta septiembre de 2004, investigamos las interacciones entre rata montera ribereñas y ratas negras para determinar si las interacciones competitivas han reducido la abundancia de ratas monteras o el éxito reproductivo. Se identificaron dos sitios con ratas monteras ribereñas en CMSP. Entre enero de 2003 y septiembre de 2004 y se retiraron 179 ratas negras de un sitio. Se evaluó la abundancia de ambas especies y el éxito reproductivo de las ratas monteras mediante trampas vivas y radiotelemetría. l tamaño promedio de camada, el promedio de crías emergidas por camadasy el promedio de crías por hembra fueron más altos en los sitios con remoción ratas negras en comparación con el sitio de control. Además, el promedio de crías en las camadas con crías emergidas aumentó en el sitio de eliminación de 2003 a 2004 y disminuyó en el sitio de control en esos años. Las tendencias de abundancia de las ratas monteras fueron más equívocas y de hecho fueron mayores el sitio de control durante la mayor parte del período de eliminación de las ratas negras. Los resultados de esta investigación sugieren que las ratas negras pueden efectivamente suprimir el éxito reproductivo de las ratas monteras ribereñas, y que la eliminación de las ratas negras podría beneficiar a las ratas monteras. Las ratas negras han estado implicadas en la disminución e incluso la extinción de otros roedores nativos, incluidas otras especies de ratas monteras. Por lo tanto, las ratas negras pueden constituir una amenaza significativa para las ratas monteras ribereñas, particularmente en conjunto con otras amenazas como inundaciones, incendios forestales, la pérdida y degradación continua del hábitat. Por tanto, recomendamos que las ratas negras sean removidas trimestralmente de hábitats altamente adecuados a las ratas monteras en el CMSP. También recomendamos que se realicen estudios para identificar poblaciones adicionales de ratas monteras ribereñas y que se realicen eliminaciones de ratas negras en esas poblaciones si se justifica.
Keywords: Abundance; California; competition; endangered species; live-trapping; Neotoma fuscipes riparia; Rattus rattus; reproduction.
© 2025 Asociación Mexicana de Mastozoología, www.mastozoologiamexicana.org
Introduction
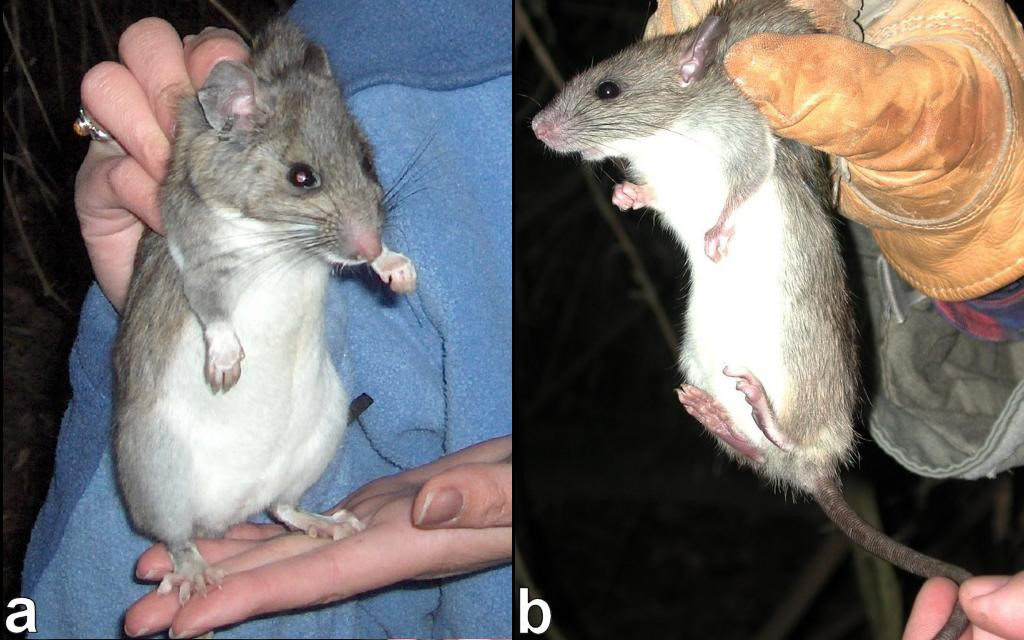
The range of the dusky-footed woodrat (Neotoma fuscipes) extends from northwestern Oregon to south-central California (California Wildlife Habitat Relationships 2024). One subspecies, the riparian woodrat (N. f. riparia), is restricted to riparian habitat in the northern San Joaquin Valley of California (Figure 1a). Due to profound loss of riparian habitat, the current range of the riparian woodrat is primarily confined to a single site, Caswell Memorial State Park (CMSP; Figure 2), comprising approximately 100 ha (250 ac) along the Stanislaus River in Stanislaus County (Williams 1986). Since 2003, riparian woodrats have also been documented periodically on the San Joaquin River National Wildlife Refuge and one or more other small populations also persist along the Stanislaus and San Joaquin Rivers (USFWS 2020). Overall, the geographic range is poorly understood. Historically, this subspecies may have occurred from the San Francisco East Bay region to Fresno County in the central San Joaquin Valley (Hooper 1938). However, recent analyses suggest that the phylogeographic relationships of woodrat populations in central California is more complicated than previously thought (Matocq 2002a, 2002b). Matocq et al. (2012) show that the CMSP population exhibits a hybrid ancestry with N. macrotis populations to the east in the Sierra Nevada. The very restricted distribution of N. f. riparia, in conjunction with various threats, resulted in the subspecies being listed as Federally Endangered in 2000 (USFWS 2000). It also is considered to be a Species of Special Concern by the State of California (Williams 1986).
Riparian woodrats occur in riparian forest habitats with an overstory dominated by valley oaks (Quercus lobata) and a dense shrub understory consisting of willow (Salix spp.), wild rose (Rosa californica), blackberry (Rubus spp.), wild grape (Vitis californica), and coyote bush (Baccharis spp.). Within these habitats, woodrats typically construct terrestrial stick nest houses measuring 0.6 to 0.9 m high and 1.2 to 1.8 m in diameter (Lindsdale and Tevis 1951; Williams 1993) but may also construct nests in the tree canopy, tree cavities, and downed logs. Each house is typically occupied by a single adult. Females produce 1 to 5 litters per year, comprising 3 to 4 young each. Reproduction can occur in all months with pregnancy rates being highest in February (Williams et al. 1992). Riparian woodrats are arboreal, primarily nocturnal, and generalist herbivores with a diet consisting of leaves, fruits, shoots, flowers, nuts, and fungi (Williams et al. 1992). Predators of riparian woodrats may include coyotes (Canis latrans), gray foxes (Urocyon cinereoargenteus), long-tailed weasels (Mustela frenata), mink (Neovison vison), raccoons (Procyon lotor), feral cats (Felis catus), bobcats (Lynx rufus), owls (Strigidae) and other raptors (Williams 1988).
Loss and degradation of riparian forest habitat is the primary cause of the decline of riparian woodrat populations and the primary threat to the continued existence of the subspecies. Over 95% of this habitat type in the Central Valley has been destroyed (Katibah 1984; Kelly et al. 2005) and by about 1980, only ~269 ha of mature riparian forest remained in the San Joaquin Valley (Williams and Kilburn 1984). Remaining old growth riparian habitat tends to be fragmented and degraded. However, since 2001 there has been a concerted effort to restore riparian habitat in some areas of the northern San Joaquin Valley (e.g., at the San Joaquin River National Wildlife Refuge). Threats to remaining habitat include flooding and wildfire (Close and Williams 1998), and CMSP has experienced multiple floods and wildfires in recent years, including a major fire in July 2022. Additional threats to the remaining riparian woodrat population include elevated predation pressure (e.g., from feral carnivores), disease, potential inbreeding depression, and demographic stochasticity (USFWS 1998). Williams (1993) estimated a peak population of 437 riparian woodrats.
An additional potential threat to riparian woodrats is competition from non-native black rats (USFWS 2000; Figure 1b). Black rats (Rattus rattus) are extremely adaptable and can readily colonize anthropogenically-altered habitats. Black rats are opportunistic omnivores and often exhibit high fecundity (Invasive Species Specialist Group 2008). Being highly arboreal, they can go anywhere that woodrats go, probably including the nest chamber of woodrat houses while the occupant is away foraging in the canopy. Consequently, black rats could potentially engage in both interference and exploitation competition with riparian woodrats. Interference competition could be in the form of spatial or temporal avoidance by woodrats, perhaps via chemical communication (Brown et al. 1996), or even direct mortality to woodrats, particularly juveniles and nestlings. Exploitation competition could be in the form of overlapping food habits or usurping of woodrat houses and food caches by black rats.
Black rats are often documented in riparian habitat throughout the Central Valley and they are abundant at CMSP. In 1993, trapping efforts at CMSP resulted in the capture of 57 riparian woodrats and 52 black rats (Williams 1993). In 2000, trapping the same areas at CMSP as in 1993, and with approximately similar levels of effort, resulted in the capture of 12 riparian woodrats and 109 black rats (CSU Stanislaus, Endangered Species Recovery Program, unpublished data). Thus, black rats appeared to have substantially increased over a short time period at CMSP.
A thriving black rat population at CMSP is a concern because, although the effects of black rats on riparian woodrats are unknown, black rats are known to significantly impact insular ecosystems worldwide (Stapp 2002; Thibault et al. 2002; Major et al. 2006; Caut et al. 2008). Also, in Florida, an inverse relationship was found between the abundance of black rats and endangered Key Largo woodrats (Neotoma floridana smalli), and no female woodrats were captured in areas occupied by black rats (Sasso and Gaines 2002). In the San Francisco Bay Area, there are concerns that non-native rats may be expanding and replacing woodrats in parts of the East Bay Regional Parks District (J. Patton, University of California-Berkeley, pers. comm.).
The overall goal of this research was to determine if black rat presence could, either directly or indirectly, be a negative influence on the reproductive success of the riparian woodrat population. The experimental strategy we applied was to compare riparian woodrat abundance and reproductive parameters between two sites, one a control site where both species were present and another site from which black rats were removed.
Materials and methods
Study area. Caswell Memorial State Park comprises approximately 104 ha near Ripon, California in San Joaquin County (Figure 2). The park lies within the floodplain of the Stanislaus River, and elevations range from 9.1 to 13.7 m. Facilities within the park include staff housing, an office building, a maintenance area, two campgrounds, and two day-use picnic areas. Riparian forest is the predominant vegetation community throughout the park. The regional climate includes mild, wet winters and hot, dry summers. Mean high and low temperatures based on data collected in nearby Stockton are 34.6 °C and 15.8 °C in July, and 11.9 °C and 3.1 °C in January. Mean annual precipitation is 354 mm, with most occurring as rain during winter (Western Regional Climate Center 2008).
Our approach for assessing the effects of black rats on riparian woodrats was to identify two sites where both species were relatively abundant. Black rats would then be removed from one site, and woodrat abundance and reproductive success would be compared between the two sites. These sites needed to be sufficiently spatially distinct to ensure minimal probability of animal movement between sites. To identify appropriate sites, exhaustive ground searches were conducted throughout CMSP to locate woodrat sign, particularly stick houses, feces, and runways. Live-trapping also was conducted to verify the presence of woodrats and black rats. The searches and trapping were conducted from September 2001 to August 2002.
In August 2002, two study sites were identified (Figure 3): Site A (Fenceline Trail) the black rat removal site, comprising approximately 11 ha, was located at the northwest edge of CMSP. Site B (Day Use Area) the control site, comprising approximately 9 ha, was about 1 km east of Site A and was located in the central portion of the park.
Dominant tree species at both sites included valley oak, California black walnut (Juglans californica), box elder (Acer negundo), and willows. The understory was dominated by blue elderberry (Sambucus mexicana), California blackberry (Rubus ursinus), salmon berry (Rubus spectabilis), and stinging nettle (Urtica diocia). Additional understory species at Site B included common figs (Ficus carica) and wild grape.
Live-trapping. Riparian woodrats and black rats were trapped to assess abundance, determine reproductive success, and, at Site A, to remove black rats. Trapping was conducted using Tomahawk live-traps (model #201, Tomahawk, Hazelhurst, Wisconsin). Traps were baited with a handful of COB horse feed (‘sweet’ COB: corn, oats, and barley mixed with molasses). A handful of polyester or cotton batting was placed in the back of each trap for nesting material. Traps were placed in wooden shelters designed to hold one or two traps, and to provide protection from inclement weather, predators, and bait-pilfering birds. Traps were opened and baited beginning about 1 hr prior to sunset and left open for at least 4 hr after sunset. Traps were occasionally left open overnight to increase capture probability.
Trapping was conducted intermittently prior to January 2003 to locate woodrat and black rat populations, and to identify study sites. From January 2003 to September 2004, trapping was conducted weekly with traps being opened for one to two nights each week in fall and winter and two to three nights each week in spring and summer. Traps were placed within 1 m of woodrat houses, by downed debris or dense vegetation actively used by woodrats, and also along active woodrat trails. Additional traps were placed randomly throughout Site A to increase the probability of capturing black rats for removal.
Captured woodrats and black rats were processed using mesh handling bags. Woodrats were handled with 1.25-cm (0.5-in) mesh bags and black rats were handled with 0.6-cm (0.25-in) mesh bags. The bags had an elastic cloth collar that fit securely around the end of a trap. The trap was then opened to allow the animal to enter the bag. Animals were restrained in the bag during processing. Data collected for both species included sex, mass, and age. Each individual received a uniquely numbered ear tag. Woodrats were further marked with passive integrated transponder (PIT) tags inserted subcutaneously, and a tissue sample was collected from an ear with a biopsy punch for genetic analyses. Animals were released at their capture locations. Fecal samples were collected from traps for diet analyses. Mean weights were compared between riparian woodrats and black rats by sex using two-tailed t-tests.
Black rat removal. From January 2003 to September 2004, 179 black rats were removed from Site A. Black rats were captured during routine live-trapping activities, as described above. Captured black rats were euthanized by injecting an overdose (2 cc) of Pentobarbital Sodium or T-81 (3 cc) into stomach muscles. Carcasses of black rats were collected, labeled, and frozen for future analyses.
Radio telemetry. Radio telemetry was used on some individuals of both species. To minimize risk to the woodrats (required due to their endangered status), radio-collaring techniques and protocols were developed by trapping and collaring non-endangered woodrats (n = 4) at the San Joaquin Experimental Range in Fresno County between 8 May and 24 June, 2002.
At CMSP, radio-collaring was conducted from January 2003 to September 2004. Individuals to be collared were placed in a large glass jar (ca. 25 cm tall and 14 cm in diameter) that had attached to a tight-fitting lid a cotton pad that was saturated with three ml of Isoflurane. Once fully sedated and immobile, the animals were removed from the jar for radio-collar attachment. If animals recovered mobility before collaring was completed, up to two additional attempts were made to re-sedate the animal, with an additional 1.5 ml of Isoflurane added on the third attempt. If an animal could not be successfully collared after three attempts, collaring was discontinued and the animal was released. Animals that had been sedated were placed back in traps for at least 20 min to allow them to regain full mobility before being released at the capture site.
Radiocollars (Biotrack Ltd, Dorset, UK) comprised a transmitter affixed to a cable tie-type collar. To prevent snagging, the antenna was wound around the cable tie and secured to it with heat-shrink wrap. The entire unit weighed about 6 g and was < 5% of each animal’s body weight. The transmitter signal had a range of 200-600 m.
During the study, 23 woodrats were radio-collared at Site A and 24 were collared at Site B. In addition, 11 black rats were radio-collared at Site B. Radio-collared woodrats and black rats were tracked at least once each week using a telemetry receiver (AVM, Colfax, California and Advanced Telemetry Systems, Isanti, Minnesota) and a two-element “H” antenna (Telonics, Mesa, Arizona). Tracking was conducted primarily during the afternoon, just prior to trap setting. Animals were tracked to their houses or other resting areas. Telemetry locations were used for targeted trapping of female woodrats to assess reproductive condition and to determine whether black rats were using or visiting known woodrat houses or natal dens.
Woodrat Reproductive Success. To assess reproductive success, we examined several reproductive performance variables for riparian woodrats. We conducted live-trapping on a weekly basis to determine the reproductive status of adult females and to identify young produced by each female. Pregnant females exhibited signs such as a slightly swollen abdomen, a perforate vulva, and pink, loose skin with longitudinal wrinkles in the mammary area. Pregnant females also exhibited a slight loss of weight early in the pregnancy, a gain of >30 g towards the end of pregnancy, and a sudden weight loss upon giving birth (Linsdale and Tevis 1951). These criteria were used to identify reproductive females and estimate the number of litters that each individual produced each year.
Neonatal woodrats remain in their natal dens (usually stick houses) for the first 6-8 weeks of life. Because of the construction and complexity of woodrat houses, accessing nest chambers is difficult, highly invasive, and usually destructive; further, it could place the occupants at risk of injury, abandonment, or predation. Consequently, estimates of reproductive success and the number of young produced by adult females were based on the capture of juveniles following emergence from their natal dens. Juveniles were detected by live-trapping near the houses of females that were determined to have been pregnant and to have produced young. Commonly, multiple traps (2 to 4, or more) were placed near the houses in the hopes of capturing the adult female and multiple young. Individuals weighing less than 200 g at first capture were considered to have been born during the current breeding season. As is common with many small mammals, during their first few months of life, dusky-footed woodrats exhibit a linear growth rate. For a coastal subspecies (Neotoma fuscipes luciana), Kelly (1990) aged juvenile woodrats based on their weight at first capture by applying the following formulae:
Males: Age AFC = (weight AFC – 13)/2.167.
Females: Age AFC = (weight AFC – 13.5)/1.828.
where AFC is “At First Capture” and weights are recorded in grams.
One or more of the following criteria were used to assign young to specific adult females:
1. Estimated age of the juvenile corresponded with the estimated date of parturition for the female;
2. Young and an adult female were captured in the same trap or at the same trap station;
3. Young were captured in the same trap or at the same trap station as other known young of a given female;
4. Young were not captured with a female, but were captured at least three times near the house of a lactating female.
In one case, two natal dens were in close proximity to each other and the females associated with these dens had both given birth at about the same time. Thus, the three young captured in this area could not be confidently associated with either female, and therefore 1.5 young were assigned to each female.
Data recorded for each adult female included number of pregnancies and number of emerged young per litter, which generated six variables for analysis.
Number of pregnancies per female (including those where no emerged young were documented).
Litter size: number of emerged young verified for each litter, including litters where no emerged young were recorded (i. e., litter size = 0).
Number of young per female: number of emerged young produced per year (tallied across all litters).
Number of young per pregnancy: number of emerged young for each female divided by the number of pregnancies for that female.
Number of young per litter with young: total number of young for each female divided by the number of litters with young (i. e., litter size ≥ 1) for that female.
Proportion of litters with young: number of litters with emerged young for each female divided by the number of pregnancies for that female.
For each variable, two-way analysis of variance was used to compare mean values between study sites (A and B) and between years (2003 and 2004), and to identify any interaction effect between site and year. Analyses were conducted using Statistica 6.0 (StatSoft, Inc., Tulsa, Oklahoma). Values for proportions of litters with young were transformed prior to analysis using an arcsin transformation (Zar 1984).
Abundance Estimates. Abundance estimates for riparian woodrats and black rats at each site were derived using data from live-trapping and radio telemetry. The minimum number of individuals known to be alive at each site was determined monthly for each species. This estimate was derived by tallying the number of unique individuals captured each month and adding to this the number of radio-collared individuals not captured but known to still be alive and present on the study site (based on telemetry). Individuals captured in non-consecutive months were added to tallies for all intervening months. For each species, mean abundance during the period of black rat removal was compared between sites using a paired-sample t-test.
Results
As with most rodents, woodrats and black rats exhibit sexual dimorphism, with males being larger than females. This size dimorphism is more pronounced in adult riparian woodrats (Figure 4). Both sexes are significantly larger for adult riparian woodrats compared to adult black rats (males: t39 = 12.02, P < 0.001; females: t17 = 10.18, P < 0.001; Figure 4).
Woodrat reproductive success. From January 2003 to September 2004, data on reproductive success were collected for 31 adult female riparian woodrats: 13 at Site A and 18 at Site B. At both sites, data were collected for four females in both 2003 and 2004. During the study period, 86 juvenile woodrats were captured, 70 of which (81.4 %) could be associated with individual adult females (42 at Site A, 28 at Site B).
Mean reproductive parameters were summarized by site and year (Table 1). The mean (±SE) number of pregnancies for all female woodrats was 1.59 ± 0.15 (range = 1 to 4) and did not vary between sites (F1,35 > 0.01, P = 0.99) or years (F1,35 = 0.69, P = 0.41), nor was there an interaction between site and year (F1,35 = 0.03, P = 0.86). Mean litter size was almost twice as high at Site A (1.56 ± 0.29) compared to Site B (0.80 ± 0.16), and differed significantly between sites (F1,58 = 5.69, P = 0.02); mean litter size did not differ between years (F1,58 = 1.08, P = 0.30), nor was there an interaction between sites and years (F1,58 = 0.87, P = 0.35). The mean number of young produced by each female was marginally higher (F1,35 = 3.37, P = 0.08) at Site A (2.47 ± 0.61) compared to Site B (1.27 ± 0.30), but did not differ between years (F1,35 = 1.76, P = 0.19), nor was there an interaction between sites and years (F1,35 = 0.24, P = 0.63). The mean number of young per pregnancy for all female woodrats was 1.05 (± 0.17) and did not vary between sites (F1,35 = 2.51, P = 0.12) or years (F1,35 = 0.96, P = 0.33), nor was there an interaction between site and year (F1,35 = 0.20, P = 0.66). However, consistent with observed values for the other parameters, the mean number trended higher at Site A and in 2003. The mean number of young for litters with emerged young was significantly higher (F1,35 = 8.18, P = 0.01) at Site A (2.39 ± 0.31) compared to Site B (1.51 ± 0.18), but did not differ between years (F1,35 = 0.15, P = 0.70). However, there was a marginally significant interaction between site and year (F1,35 = 3.13, P = 0.09), with the mean number of young for litters with emerged young increasing on Site A from 2003 to 2004 but decreasing at Site B (Figure 5). Finally, the mean proportion of litters with emerged young was 55.1 ± 7.2 % and did not differ between sites (F1,35 = 0.77, P = 0.39) nor was there an interaction between site and year (F1,35 = 0.27, P = 0.61). However, the mean proportion was marginally higher (F1,35 = 3.04, P = 0.09) in 2003 (67.6 ± 10.1 %) compared to 2004 (44.4 ± 9.8 %).
Abundance estimates. Monthly estimates of the minimum number of woodrats known to be present ranged from 4 to 34 at Site A and 19 to 33 at Site B (Figure 6). Mean monthly abundance of woodrats during the 21 months of black rat removal was higher (t1,20 = –4.07, P > 0.01) at Site B (27.2 ± 1.1) compared to Site A (21.1 ± 1.6). However, the patterns of abundance differed between the two study sites. Both populations exhibited an increase during May to July 2003, which likely was associated with reproduction and the presence of emerged young. At Site A, abundance decreased again in August 2003 and remained at a lower level until increasing again in April 2004 in association with annual reproduction. Abundance then declined from July 2004 until the end of the study in September 2004. Conversely, after increasing in spring 2003, abundance at Site B remained at a consistently high level through June 2004, after which abundance declined monthly through the end of the study, similar to that observed at Site A.
Black rat abundance also differed between the two study sites (Figure 6). At Site B, monthly estimates of the minimum number known alive ranged from 0 to 28, peaking each spring and summer coincident with annual reproduction. Interestingly and for unknown reasons, the population declined precipitously after July 2004 and no black rats were captured at Site B in the last month of the study. At Site A, abundance estimates ranged from 3 to 28. Black rat removals began in January 2003, and abundance declined markedly by April 2003. Abundance increased again during May-July 2003, probably as a result of reproduction, but then plummeted in August and stayed at a relatively low level for the remainder of the study. After July 2003, abundance estimates remained at 10 or lower. This suggests that removal efforts were successful in markedly reducing black rat abundance at Site A. Mean monthly abundance during the 21-month removal effort was significantly higher (t1,20 = –3.33, P > 0.01) at Site B (15.9 ± 1.5) compared to Site A (9.6 ± 1.7).
Discussion
Interactions between endangered riparian woodrats and non-native black rats at Caswell Memorial State Park were investigated for a 37-month period (September 2001 to September 2004), with the response of woodrats to black rat removal being investigated for 21 of those months (January 2003 to September 2004). The removal of black rats from Site A (Fenceline Trail) appeared successful in reducing black rat abundance at that site. This was a consequence of several months of trapping, but from late summer 2003 until the end of the study, black rat abundance remained consistently lower at Site A than at Site B (Day Use Area).
Of six parameters of reproductive success examined for woodrats, two (mean litter size and mean number of young per litter-with-young) were significantly higher and another (mean number of young per female) was marginally higher on the black rat removal site. Furthermore, the mean number of young in litters with young increased substantially between years at Site A, but exhibited a concomitant decrease at Site B, from which black rats were not removed. Collectively, these results suggest that woodrat reproductive success was higher at Site A. Although the positive response at Site A cannot be conclusively attributed to the removal of black rats, the implied correlation is compelling.
Reduced black rat abundance may have benefited woodrat reproductive success in several ways. Through exploitative competition, black rats may have been reducing the availability of resources (e.g., food, houses) to woodrats. Nutritionally stressed woodrats likely would produce fewer offspring, either through pregnancy failure, reduced litter size, or reduced juvenile survival. If woodrats were being displaced from houses, reproductive success could be lowered through exposure of young to the elements (e.g., precipitation, cool nighttime temperatures) or increased predation rates due to a lack of adequate shelter. However, we have no evidence to suggest that black rats can exploit resources essential to woodrat survival. Adult woodrats are larger than adult black rats, and woodrats are known to aggressively defend resources (Linsdale and Tevis 1951), so we believe that in one-on-one interspecific encounters, adult woodrats (of either sex) would dominate adult black rats.
Interference competition from black rats could also have reduced woodrat reproductive success. Although we did not produce any data in support of this, black rats have the potential to prey on woodrat young, thereby causing direct mortality; they could prey on nestling young while the mother is out foraging or they could potentially prey on or harass and drive off emergent young. Even if direct predation is not occurring, harassment by black rats or even just their presence, especially if the black rat population density was as high or higher than the woodrat population, could negatively affect woodrat reproductive success. For example, if woodrats were spending inordinate amounts of time repelling black rats, then less time would have been available for attending young or feeding to maintain a sufficient nutritional plane to rear young (e.g., energy for lactation). In southeastern Australia, Stokes et al. (2009) concluded that black rats were reducing reproductive success of native bush rats (Rattus fuscipes), apparently through interference competition with juvenile bush rats.
Although black rat and woodrat densities were not markedly different at the Day Use Area (Site B), long-term trapping data for CMSP indicates that black rat density can be significantly higher than woodrat density. Furthermore, the presence of black rats could constitute an environmental stressor for woodrats. Chronically elevated glucocorticoid steroid hormones associated with persistent stressors can produce a suite of deleterious physiological effects including reproductive suppression (Sapolsky et al. 2000). Thus, removal of black rats could have reduced competitive pressure and stress experienced by woodrats.
The effect of black rat removal on the abundance of riparian woodrats was more equivocal. Despite the reduction in black rat abundance at Site A, woodrat abundance at this site was typically lower than that at Site B. Not until the last five months of the study did woodrat abundance on Site A equal and then exceed that at Site B.
It is possible that the black rat population at CMSP is a consequence of it being a state park, and in particular because it has a campground as well as day use areas. From ESRP trapping results through 2005, black rats do appear to be more common in the campground area but they are also quite common in other areas of the park, including relatively isolated areas such as along Fenceline Trail. Further, black rats are known to be common in other riparian areas throughout the San Joaquin Valley. Here we provide just two examples of their pervasiveness in natural areas. Since its initiation in 2002, black rats have been frequent captures in the riparian brush rabbit (RBR, Sylvilagus bachmani riparius) recovery implementation program at the San Joaquin River National Wildlife Refuge and other nearby RBR release sites (Faith Ranch, Buffington Tract; latter is across the Stanislaus River from CMSP). Also, between 2004 and 2008, an average of 124 black rats were captured and removed annually from the RBR breeding pens near Lodi, an area that has very little human disturbance or anthropogenic food sources (except perhaps nearby croplands, although little appears to be known about the extent of use of croplands by Rattus in California).
The results of this investigation are not definitive. Certain intrinsic and extrinsic attributes of the data may have precluded detection of differences between our sites, and possibly confounded our results. For example, it is possible that a positive response by woodrats might have become more pronounced with a longer period of black rat removal. The removal in this study was conducted for just 21 months. A cause-and-effect relationship might have been more strongly supported with a longer study. Also, there may have been environmental differences (e.g., habitat suitability, food availability) between sites that might have affected differences in the response by woodrats to black rat removal. Annual environmental variation and even stochastic demographic variation may have contributed to this effect.
All of the above would have rendered the detection of differences between the two study sites more difficult. Despite this, the results did provide strong evidence of certain trends, most of which indicated that the removal of black rats conferred a benefit to woodrats. However, two cautions are in order. Autocorrelation among some of the reproductive parameters could have inflated the number of parameters exhibiting differences. Also, a marked increase in the abundance of raccoons, a potential predator of woodrats, in spring 2004 at Site B could have contributed to the decrease in woodrat reproductive success between 2003 and 2004 at that site.
Black rats are native to the Indian sub-continent but have been unintentionally spread by humans to all continents except Antarctica. Due to immense ecological plasticity, they can occupy a diversity of habitats and consume a wide range of foods consisting of plants, fungi, and animals. Consequently, black rats have caused or contributed to the extinction of various species of birds, small mammals, reptiles, invertebrates, and plants (Invasive Species Specialist Group 2008). Catastrophic declines of many bird populations attributable to black and Norway rats (Rattus norvegicus) are well documented (e.g., Atkinson 1985; Thibault et al. 2002; Major et al. 2006; Invasive Species Specialist Group 2008).
Black rats have adversely impacted native rodents, including rare species, in other ecosystems worldwide, particularly insular ones. In the Galapagos Islands, black rats either directly caused or contributed to the extinctions of three of seven endemic rice rat species (Oryzomys spp. and Nesoryzomys spp.). Of the four extant species, the Santiago rice rat (N. swarthi) was presumed extinct, but small numbers were rediscovered on Santiago Island in 1997 (Dowler et al. 2000). Removals of black rats on this island significantly slowed the rate of population decline of rice rats, primarily through increased survival of female rice rats (Harris and Macdonald 2007). Similarly, populations of rare birds and snakes increased on Antiguan islands following the removal of black rats (Daltry et al. 2012). Finally, as mentioned previously, black rats have also been implicated in the decline of the endangered Key Largo woodrat in Florida (Sasso and Gaines 2002).
Our study strongly suggests that black rats also can have impacts in a non-insular setting. Similarly, black rats were found to be adversely affecting the native bush rat in rainforest habitat in southeastern Australia (Stokes et al. 2009). Bush rat populations increased significantly following removal of black rats, and the latter did not re-establish after removal as the increase in bush rats, particularly adult females, apparently shifted the competitive advantage to bush rats.
The results of this investigation indicate that black rats may have adverse impacts on riparian woodrat populations. The woodrat population at CMSP had been recognized as the only extant population for this subspecies (USFWS 1998). While there is little recent information on woodrat abundance in the park, there is increased concern about the status of the population. A recent ground search (~2 hours on April 18, 2024) for woodrat sign (houses, vegetation clippings, droppings) could not confirm woodrat presence. However, an adult riparian woodrat was captured on video during vegetation management activities on October 29, 2024 (C. Bradley, California State Parks, pers. comm.). In March 2003, a putative riparian woodrat was captured at the San Joaquin River National Wildlife Refuge (SJRNWR) about 8 km south of CMSP and over 30 additional individuals have been captured since (CSU Stanislaus, Endangered Species Recovery Program, unpublished data). Genetic analyses of samples from these individuals indicated that similar to the woodrats at CMSP, the ones at SJRNWR include an admixture of genes from N. fuscipes and N. macrotis (Matocq et al. 2012). However, even if the SJNWR population is classified as the riparian woodrat, it would constitute only a second population for the subspecies, and having just two recognized populations would still leave this subspecies with a very restricted distribution and extremely vulnerable to catastrophic events (e.g., wildfire, floods, disease). Thus, any reasonable efforts should be employed to conserve these populations. These efforts could include black rat control or removal.
Removing black rats on a sustained, long-term basis would no doubt be challenging, and complete removal is probably impossible. Black rats exhibit high reproductive rates and rapid dispersal and in central California they occur in urban areas and in habitats having dense vegetation, especially riparian habitat. Thus, accessing black rats is difficult and removed rats might be quickly replaced through reproduction or immigration. Furthermore, unlike some other locations where large-scale broadcasting of rodenticide-laced baits has been used to reduce or eliminate black rats (e.g., Howald et al. 2005), impacts to non-target species, as well as the presence of endangered woodrats, negates use of this strategy. Thus, live-trapping and euthanizing black rats currently is the only practical removal method that minimizes risk to woodrats. However, this strategy is labor intensive and therefore expensive.
Another potential strategy to help control black rat abundance might be the reintroduction of ringtails (Bassariscus astutus) to CMSP and other riparian areas of the northern San Joaquin Valley. Recent research in the Sacramento Valley has indicated that ringtails prey heavily on black rats (D. Wyatt, Sacramento City College, pers. comm). Provided that ringtail reintroduction would not adversely impact riparian woodrat and riparian brush rabbit populations, this may be a useful strategy to consider. We would expect ringtails to prey on woodrats, and possibly also brush rabbits, but if they differentially prey on black rats, that could result in a competitive advantage for woodrats (and brush rabbits) over black rats.
Finally, one strategy that might be practical and cost-efficient is to identify a set of “core areas” that encompass optimal habitat for woodrats and then focus black rat removal efforts on only those areas. Each area should be sufficiently large to support a sizeable woodrat population, but also sufficiently small such that black rat numbers could be effectively reduced via a standardized live-trapping program, especially during the woodrat breeding season, along established trap lines or trapping stations. Depending on habitat parameters (woodrats can reach high densities under optimal habitat conditions), an area of 10-20 ha might be sufficient to achieve these objectives. Valuable information as well as cost efficacy could be gained by also using these trapping efforts to monitor the woodrat population.
Recommendations. Based on our results, we offer the following recommendations.
A minimum of two core areas of 10 to 20 ha in size, with high quality habitat and high density woodrat populations, should be identified at CMSP.
A black rat removal program should be implemented within these core areas.
Conducting removals multiple times per year would be most effective, but minimally removals should be conducted in the 3 months (January to March) prior to the woodrat breeding season to minimize impacts by black rats on woodrat reproductive success.
Explore the use of emerging fertility control methods for black rats. Such methods would need to be specific to black rats to avoid impacting woodrats.
Surveys should be conducted in locations other than CMSP to identify additional riparian woodrat populations.
If black rats are present, as expected, in any newly identified riparian woodrat populations, then a removal program, similar to the one proposed at CMSP, should also be conducted in those locations.
In all locations with riparian woodrats, a concerted effort should be made to remove or reduce anthropogenic sources of food and shelter for black rats.
Until additional riparian woodrat populations are found, a captive colony of riparian woodrats should be established, particularly given the myriad threats (e.g., black rats, flooding, wildfire, disease, predators) to the CMSP population.
In addition to serving as insurance against a catastrophic event at CMSP, a captive colony could be used to breed riparian woodrats for introduction into areas with suitable vacant habitat.
The reintroduction of ringtails to CMSP and other riparian areas of the northern San Joaquin Valley should be investigated further. Successful reintroduction of ringtails could not only benefit riparian woodrats and riparian brush rabbit, but it would also restore a semblance of the more complete riparian community that existed in the 19th and early 20th centuries.
Acknowledgments
Trapping and handling were conducted in accordance with protocols established in a recovery permit from the U.S. Fish and Wildlife Service (TE023496) and a Memorandum of Understanding with the California Department of Fish and Game. All field methods were consistent with guidelines for the use of wild animals in research established by the American Society of Mammalogists (Sikes and Animal Care and Use Committee of the American Society of Mammalogists 2016). Many people assisted with the fieldwork (trapping, telemetry, etc.). In particular, we would like to thank K. Rosado, S. Deal, M. Hopkins, and others for their extensive assistance in the field, and M. Lloyd for his assistance in analysis and report development. S. Kelly prepared the Spanish abstract. For financial support of this research, we thank the Central Valley Project Conservation Program and Habitat Restoration Program, the U.S. Bureau of Reclamation, the California Department of Fish and Game, and the U.S. Fish and Wildlife Service. We thank the California Department of Parks and Recreation for assistance (including camping space and electricity supply access), logistical support, and access to Caswell Memorial State Park (including permits). We gratefully acknowledge the commitment, support, and assistance provided to the project by the following: R. Faubion, C. Solomon, and J. Tomson (U.S. Bureau of Reclamation); R. Schlorff (California Department of Fish and Game); H. McQuillen, C. Prose, and A. Willy (U.S. Fish and Wildlife Service); J. Karlton and T. Jensen (California Department of Parks and Recreation). We thank M. Matoq and an anonymous reviewer for comments that significantly improved the manuscript.
The authors would like to acknowledge the myriad contributions of Jim Patton to our knowledge of mammals, in particular our understanding of the ecology and diversity of New World mammals. We also acknowledge the tremendous role played by Carol Patton, Jim’s lifelong field partner, in this great body of work. Finally, we thank them both for their friendship, as well as their mentorship of so many students over the years.
Literature cited
Atkinson, I. A. E. 1985. The spread of commensal species of Rattus rattus to oceanic islands and their effects on island avifauna. Pp. 35-81, in Conservation of island birds (Moors, P. J., ed.) International Council for Bird Preservation, Cambridge, U.K.
Begon, M., and M. Mortimer. 1986. Population Ecology: A Unified Study of Animals and Plants. Sinauer Associates, Inc. Sunderland, Massachusetts, U.S.A.
Brown, K. P., et al. 1996. Calibration of tunnel tracking rates to estimate relative abundance of ship rats (Rattus rattus) and mice (Mus musculus) in a New Zealand forest. New Zealand Journal of Ecology 20:271-275.
California Wildlife Habitat Relationships. 2024. Range map for Neotoma fuscipes. https://nrm.dfg.ca.gov/FileHandler.ashx?DocumentID=2524&inline=1. Accessed August 2024.
Caut, S, E. Angulo, and F. Courchamp. 2008. Dietary shift of an invasive predator: rats, seabirds and sea turtles. Journal of Applied Ecology 45:428-437.
Close, C. L., and D. F. Williams. 1998. Habitat management for riparian brush rabbits and woodrats with special attention to fire and flood. Final report to the U.S. Bureau of Reclamation, South-Central California Area Office, Fresno, California, U.S.A. http://esrp.csustan.edu/publications/pdf/esrp_1998_rbr-fire-flood.pdf
Daltry, J. C., et al. 2012. Evidence that eradicating black rats has boosted the recovery of rare reptiles and seabirds on Antiguan islands. Proceedings of the conference Biodiversité Insulaire: la flore, la faune et l’homme dans les Petites Antilles. Pp. 146-157. https://www.martinique.developpement-durable.gouv.fr/IMG/pdf/Mieux_connaitre_la_biodiversite2_cle724bcb.pdf#page=146.
Dowler, R. C., D. S. Carroll, and C. W. Edwards. 2000. Rediscovery of rodents (Genus Nesoryzomys) considered extinct in the Galapagos Islands. Oryx 34:109-117.
Harris, D. B., and D. W. Macdonald. 2007. Interference competition between introduced black rats and endemic Galapagos rice rats. Ecology 88:2330-2344.
Hooper, E. T. 1938. Geographical variation in wood rats of the species Neotoma fuscipes. University of California Publications in Zoology 42:213-246.
Howald, G. R., et al. 2003. Eradication of black rats from Anacapa Island: biological and social considerations. Pp. 299-312, in Proceedings of the sixth California Islands symposium (Garcelon, D. K., and C. A. Schwemm, eds.). National Park Service Technical Publication CHIS-05–01. Institute for Wildlife Studies, Arcata, California, U.S.A.
Invasive Species Survival Group. 2008. Global invasive species database, Rattus rattus. http://www.issg.org/database/species/ecology.asp?si=19&fr=1&sts=sss. Accessed March 2008.
Katibah, E. F. 1984. A Brief History of Riparian Forests in the Central Valley of California. Pp. 23-29, in California riparian systems: ecology, conservation, and productive management (Warner, R. E., and K. M. Hendrix, eds.). University of California Press. Berkeley, U.S.A.
Kelly, P. A. 1990. Population ecology and social organization of dusky-footed woodrats, Neotoma fuscipes. Ph.D. dissertation, University of California, Berkeley, U.S.A.
Kelly, P. A., S. E. Phillips, and D. F. Williams. 2005. Documenting ecological change in time and space: the San Joaquin Valley of California. Pp. 57-78, in Mammalian diversification: from chromosomes to phylogeography (Lacey, E. A. and P. Myers, eds.). University of California Press. Berkeley, U.S.A.
Linsdale, J. M., and L. P. Tevis, Jr. 1951. The dusky-footed wood rat. University of California Press. Berkeley, U.S.A.
Major, H. L., et al. 2006. Assessing the effects of introduced Norway rats (Rattus norvegicus) on survival and productivity of least auklets (Aethia pusilla). The Auk 123:681–694.
Matocq, M. D. 2002a. Morphological and molecular analysis of a contact zone in the Neotoma fuscipes species complex. Journal of Mammalogy 83:866-883.
Matocq, M. D. 2002b. Phylogeographical structure and regional history of the dusky-footed woodrat, Neotoma fuscipes. Molecular Ecology 11:229-242.
Matocq, M. D., et al. 2012. Reconstructing the evolutionary history of an endangered subspecies across the changing landscape of the Great Central Valley of California. Molecular Ecology 21:5918-5933.
Pergams, O. R. W., R. C. Lacey, and M. V. Ashley. 2000. Conservation and management of Anacapa Island deer mice. Conservation Biology 14:819-832.
Sapolsky, R. M., L. M. Romero, and A. U. Munck. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews 21:55-89.
Sasso, C. R., and M. S. Gaines. 2002. Population ecology of three species of small mammals on Key Largo, Florida. Florida Scientist 65:115-125.
Sikes, R. S., and Animal Care and Use Committee of the American Society of Mammalogists. 2016. Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy 97:663-688.
Stapp, P. 2002. Stable isotopes reveal evidence of predation by ship rats on seabirds on the Shiant Islands, Scotland. Journal of Applied Ecology 39:831-840.
Stokes, V. L., et al. 2009. Competition in an invaded rodent community reveals black rats as a threat to native bush rats in littoral rainforest of south-eastern Australia. Journal of Applied Ecology 46:1239-1247.
Thibault, J.-C., et al. 2002. Understanding the decline and extinction of monarchs (Aves) in Polynesian Islands. Biological Conservation 108:161-174.
U.S. Fish and Wildlife Service (USFWS). 1998. Recovery plan for upland species of the San Joaquin Valley, California. U.S. Fish and Wildlife Service. Portland, U.S.A.
U.S. Fish and Wildlife Service (USFWS). 2000. Endangered and threatened wildlife and plants; final rule to list the riparian brush rabbit and the riparian, or San Joaquin Valley, woodrat as endangered. Federal Register 65:8881-8890.
U.S. Fish and Wildlife Service (USFWS). 2020. 5-year review: Riparian woodrat (Neotoma fuscipes riparius). U.S. Fish and Wildlife Service. Sacramento, California, U.S.A.
Western Regional Climate Center. 2008. Climatological data summary for Stockton WSO, California. http://www.wrcc.dri.edu/cgi-bin/cliMAIN.pl?ca8558. Accessed March 2008.
Williams, D. F. 1986. Mammalian species of special concern in California. California Department of Fish and Game, Wildlife Management Division, Administrative Report 86-1. Sacramento, U.S.A.
Williams, D. F. 1988. Ecology and management of the riparian brush rabbit in Caswell Memorial State Park. California Department of Parks and Recreation, Interagency Agreement Final Report 4-305-6108. Lodi, U.S.A.
Williams, D. F. 1993. Population censuses of riparian brush rabbits and riparian woodrats at Caswell Memorial State Park during January 1993. Final Report to California Department of Parks and Recreation. Lodi, U.S.A.
Williams, D. F., and K. S. Kilburn. 1984. Sensitive, threatened, and endangered mammals of riparian and other wetland communities in California. Pp. 950-956, in California riparian systems ecology, conservation, and productive management (Warner, R. E., and K. M. Hendrix, eds.). University of California Press. Berkeley, U.S.A.
Williams, D. F., et al. 1992. General biology of major prey species of the California spotted owl. USDA Forest Service General Technical Report PSW-GTR-133:207-221.
Zar, J. H. 1984. Biostatistical analysis. Second edition. Prentice-Hall, Inc. Englewood Cliffs, New Jersey, U.S.A.
Associated editors: Marjorie Matocq and Eileen Lacey
Submitted: October 20, 2024; Reviewed: November 3, 2024
Accepted: November 26, 2024; Published on line: January 31, 2025

Figure 1. a. Riparian woodrat (Neotoma fuscipes riparia); b. Black rat (Rattus rattus).
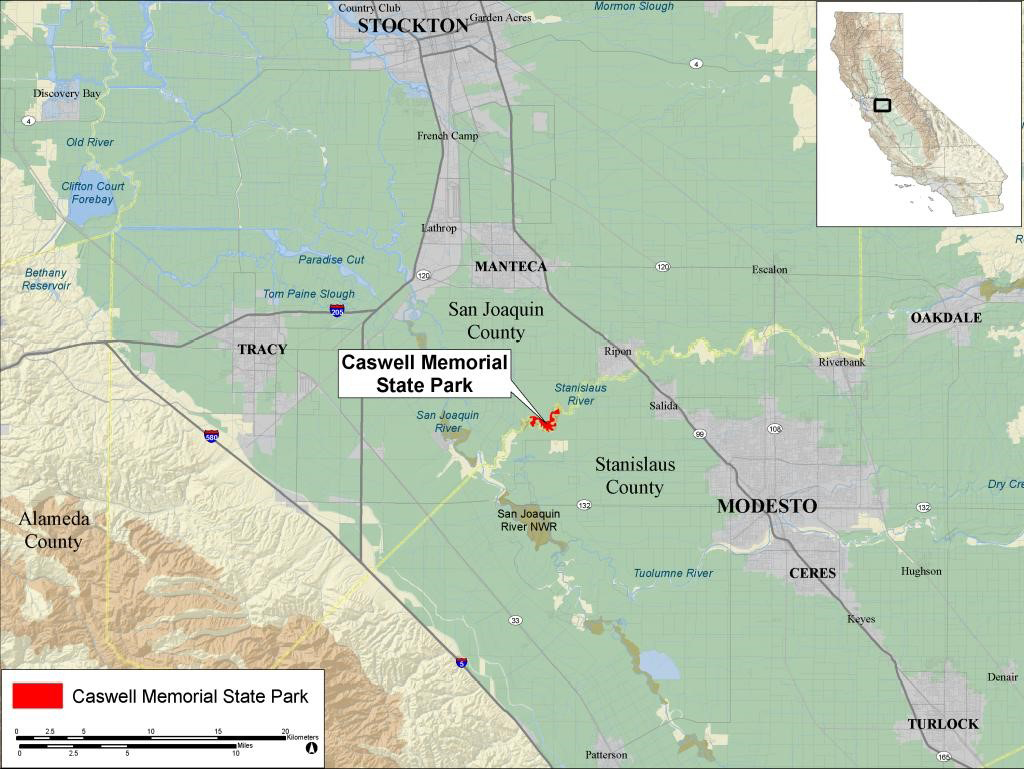
Figure 2. Location of Caswell Memorial State Park, California.
.jpg)
Figure 3. Black rat removal site (A: Fenceline Trail) and control site (B: Day Use Area), Caswell Memorial State Park, California.
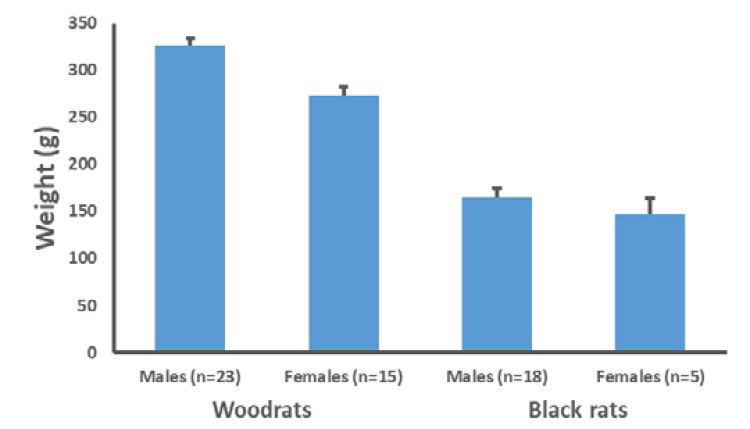
Figure 4. Mean body mass with standard error bars for adult riparian woodrats and black rats at Caswell Memorial State Park, California.
Table 1. Reproductive parameters (mean and standard error) by study site and year for female riparian woodrats at Caswell Memorial State Park, California, from January 2003 to September 2004.
|
Site1/ Year |
No. females |
Pregnancies per female |
Litter size |
Young per female |
Young per pregnancy |
Young per litters with young |
Percent litters with young |
|
Site A |
17 |
1.6 (0.2) |
1.6 (0.3) |
2.5 (0.6) |
1.4 (0.3) |
2.4 (0.3) |
61.3 (0.1) |
|
Site B |
22 |
1.6 (0.2) |
0.8 (0.2) |
1.3 (0.3) |
0.8 (0.2) |
1.5 (0.2) |
50.4 (0.1) |
|
2003 |
18 |
1.7 (0.3) |
1.3 (0.2) |
2.3 (0.5) |
1.2 (0.2) |
1.9 (0.2) |
67.6 (0.1) |
|
2004 |
21 |
1.5 (0.2) |
0.9 (0.2) |
1.4 (0.4) |
0.9 (0.3) |
1.9 (0.3) |
44.4 (0.1) |
|
Total |
39 |
1.6 (0.2) |
1.1 (0.2) |
1.8 (0.3) |
1.1 (0.2) |
1.9 (0.2) |
55.1 (0.1) |
1 Site A. Fenceline Trail, black rats removed. Site B. Day Use Area, black rats not removed.
.jpg)
Figure 5. Mean number of young with standard error bars for litters with emerged young on the black rat removal site (A: Fenceline Trail) and control site (B: Day Use Area), Caswell Memorial State Park, California, from January 2003 to September 2004.
Figure 6. Minimum number of riparian woodrats (top) and black rats (bottom) known to be alive on two study sites—Site A - black rat removal (Fenceline Trail) and Site B - control (Day Use Area)—at Caswell Memorial State Park, California, from January 2003 to September 2004.
.jpg)