THERYA, 2025, Vol. 16(1):175-183 DOI:10.12933/therya-25-6176 ISSN 2007-3364
Eavesdropping on bats in Peninsular Thailand: a trial application of automated recorders to monitor habitat changes
Grace Rui-Tong Yu1, Ariya Dejtaradol2, Yingyod Lapwong2, Sunate Karapan3, Pipat Soisook4, Chia-Yun Lee5, Mao-Ning Tuanmu5, Hon-Tsen Yu6 *
1 Department of Biology, University of British Columbia Okanagan, Kelowna. British Columbia, Canada V1V 1V7. Email: grace.yu945@gmail.com (GR-TY). Current address: Kang-Chiao International School, Xin-Dian District, New Taipei City. Taiwan ROC 231.
2 Division of Biological Science, Faculty of Science, Prince of Songkla University, Hat Yai. Songkhla, Thailand 90110. Email: ariya.d@psu.ac.th (AD), yingyod.l@psu.ac.th (YL).
3 Halabala Wildlife Research Station, Wildlife Research Division, Wildlife Conservation Bureau, Department of National Parks, Wildlife and Plant Conservation. Narathiwat, Thailand, 90160. Email: karapann@gmail.com (SK).
4 Princess Maha Chakri Sirindhorn Natural History Museum, Prince of Songkla University, Hat Yai. Songkhla, Thailand, 90110. Email: pipat.s@psu.ac.th (PS).
5 Biodiversity Research Center, Academia Sinica, Taipei. Taiwan, ROC 115. Email: janiceli0918@gmail.com (C-YL), mntuanmu@gate.sinica.edu.tw (M-NT).
6 Department of Life Science, National Taiwan University, Taipei. Taiwan, ROC 106. Email: ayu@ntu.edu.tw (H-TY).
*Corresponding author: ayu@ntu.edu.tw.
We deployed automated bat recorders for one week in the southern part of Peninsular Thailand in an attempt to monitor bat species diversity and activities. Two sites were chosen: one on a forested slope adjacent to urban development, the campus of Prince of Songkla University (PSU), and the other in a natural tropical rainforest, the Hala-Bala Wildlife Sanctuary (Bala). From PSU, we analyzed 9,744 5s time windows of recordings that were obtained from the dry season (June 2023) and the wet season (October 2023); from Bala, we analyzed 4,692 5s time windows in the wet season (October 2023). Among a total of 14,436 time windows, we detected bat acoustic signals in 1986 (13.8 %) representing 10 species of bat: eight species at PSU and six species at Bala. The recordings permitted analyses of diel activity patterns for the four species with the most acoustic records, as well as estimates of relative species abundance in accordance with forest type and season. Our results demonstrate that using automated bat recorders can help unravel bat diversity, activity patterns, and the potential for interspecific interactions. Nonetheless, independent efforts to collect and verify acoustic signals by catching and observing live bats are needed to ensure accurate species identification.
Se utilizaron grabadoras automáticas de llamados de murciélagos durante una semana en la parte sur de la península de Tailandia en un intento de monitorear la diversidad y las actividades de las especies. Se eligieron dos sitios: uno en una ladera boscosa adyacente al desarrollo urbano, el campus de la Prince of Songkla University (PSU), y el otro en una selva tropical natural, el Santuario de Vida Silvestre Hala-Bala (Bala). De PSU, analizamos 9,744 ventanas de tiempo de 5 s de grabaciones que se obtuvieron de la estación seca (junio de 2023) y la estación lluviosa (octubre de 2023); de Bala, analizamos 4,692 secciones de tiempo de 5 s en la estación lluviosa (octubre de 2023). Entre un total de 14,436 secciones de tiempo, detectamos señales acústicas de murciélagos en 1986 (13.8 %) que representan 10 especies de murciélagos: ocho especies en PSU y seis especies en Bala. Las grabaciones permitieron realizar análisis de los patrones de actividad diurna de las cuatro especies con más registros acústicos, así como estimaciones de la abundancia relativa de especies según el tipo de bosque y la estación. Nuestros resultados demuestran que el uso de grabadoras automáticas de murciélagos puede ayudar a desentrañar la diversidad de murciélagos, los patrones de actividad y el potencial de interacciones interespecíficas. No obstante, se necesitan esfuerzos independientes para recopilar y verificar señales acústicas mediante la captura y observación de murciélagos vivos para garantizar una identificación precisa de las especies.
Keywords: Acoustic signals; automated recorder; bat; diel activity pattern; habitat monitor; peninsular Thailand.
© 2025 Asociación Mexicana de Mastozoología, www.mastozoologiamexicana.org
Introduction
Sensor technologies, including automatic bat recorders, are increasingly used for passive monitoring of biodiversity and for ecological surveillance (Sethi et al. 2020; Yoh et al. 2022). The prolonged and consecutive nature of such recordings offers insights into animal activity at a much greater level of detail than previously possible, especially for echolocating bats, which are primarily nocturnal and often secretive. Furthermore, high-frequency echolocating sounds emitted by bats residing in a particular habitat should be identifiable and distinguishable from those of bat assemblages (sonotypes and their relative abundances) in other habitat types. Bat ultrasonic signals should be amenable to this purpose because bat foraging behaviors are known to adapt to prey and habitat types (Denzinger et al. 2018; Yoh et al. 2022). In Thailand, bat calls have been analyzed to the species level (Hugh et al. 2011; Ith et al. 2011) and these data could serve as a point of reference for future studies. However, no previous studies have employed automated recorders to monitor diversity or estimate species distributions.
Peninsular Thailand is of particular interest to biogeographers because the region is situated on the northern part of the Thai-Malay Peninsula and represents a transition zone of biodiversity between the Indochinese and Sundaic faunas. Many zoogeographical studies have identified the Isthmus of Kra as a major boundary line between the two faunas, while the Kangar-Pattani Line lying ca. 500 km to the south distinctly separates northern and southern plant communities (Lohman et al. 2011). Some researchers propose that the biogeographical divergence in Peninsular Thailand resulted from repeated fluctuations in sea level over the last 5 million years rather than permanent physical barriers, such as mountains and rivers (Woodruff and Turner 2009; Woodruff 2010; Li and Li 2018). Additionally, studies focusing on avifauna have shown that changes in bird species composition near the Isthmus of Kra are linked to shifts in forest type driven by climatic factors (Hughes et al. 2003; Dejtaradol et al. 2015). A study of amphibians identified the area between the Isthmus of Kra and the Kangar-Pattani Line as a distinct biogeographic subregion called South Tenasserim (Poyarkov et al. 2021). Consequently, Peninsular Thailand is characterized as a broad biogeographical transition zone that has been shaped by multiple episodes of separation rather than a singular, sharp delineation like that represented by the Wallace Line (Hinckley et al. 2023).
Fourteen provinces are administered in Peninsular Thailand and are known collectively as the Region of Southern Thailand, with a combined area of 70,714 km2. The western part of the region features steep coastlines; on the eastern side alluvial plains predominate. The largest plain, located in Surat Thani, is formed by two rivers, the Tapi and the Phum Duang, with a total catchment of more than 8000 km2. Smaller rivers either empty into the Gulf of Thailand (e. g., the Pattani and the Saiburi) or into the Andaman Sea (e. g., the Krabi and the Trang). Additionally, Songkhla Lake (1,040 km2), the largest lake in Thailand, is a conspicuous feature and wildlife habitat in this region.
Peninsular Thailand is longitudinally divided by the southern section of the Tenasserim Range, resulting in two narrow coastal plains that experience distinct climatic conditions. The Phuket Subrange extends from the Isthmus of Kra down to Phuket Island. Approximately 100 kilometers to the east lies the Nakhon Si Thammarat, or Banthad Subrange, which begins at Phangan Island and continues southward to Songkhla Province, where it connects with the Titiwangsa Range. The majority of Peninsular Thailand belongs to the Tenasserim-South Thailand semi-evergreen rain forest ecoregion. In the adjacent region, the Peninsular Malaysian rain forest and montane rain forest ecoregions extend into southernmost Thailand (Olson et al. 2001). Today large tracts of rubber and oil palm plantations have replaced the natural forests and dominated the once forested landscape.
Thailand, as a whole, has lost 20 % of its forest cover over the last 40 years, from 53 % in 1961 to 33 % in 2000 (Bumrungsri et al. 2006), a loss of ca. 0.5 % per year. This is largely because rubber trees were introduced as a cash crop in the early 1900s and replaced native tree species. In the Region of Southern Thailand, large areas of rainforest have been converted to rubber plantations. By 1992, 25 % of the land area of Southern Thailand was occupied by rubber plantations and just 18 % remained forested. Oil palm cultivation was introduced in the 1980s (Dallinger 2011), either replacing existing rubber plantations or expanding into newly-cleared forested areas. Today, both rubber and oil palm trees dominate the landscape.
Peninsular Thailand is rich in bat diversity, containing at least 87 species of bats in eight families using laryngeal echolocation (Emballonuridae, Megadermatidae, Rhinolophidae, Hipposideridae, Vespertilionidae, Miniopteridae, Molossidae, and Nycteridae; Karapan et al. 2023). A detailed study of bats conducted by Phommexay et al. (2011) over the course of seven months contrasted bat diversity in natural rainforests with that in rubber plantations in southern Peninsular Thailand (Songkhla Province and Phatthalung Province). That study revealed a depauperate bat fauna in rubber plantations (26 species in rainforests as compared to 13 species in rubber plantations), suggesting that recent changes in land use have negatively impacted bat diversity.
We installed automated bat recorders in two forested areas in Southern Thailand, aiming to test their feasibility in monitoring the impact of the habitat changes on bat diversity caused by deforestation and cultivation of economically-important plants. Specifically, we chose a natural reserve area (Bala site) in which original forests remain intact and a site (PSU) where rubber trees had been planted but were later abandoned and ceased to be managed as plantations, permitting secondary forests to regrow. Bat acoustic signals were recorded and analyzed to examine the differences in bat diversity, behavior and activity patterns in relation to the differences in habitat and land use between these sites.
Materials and methods
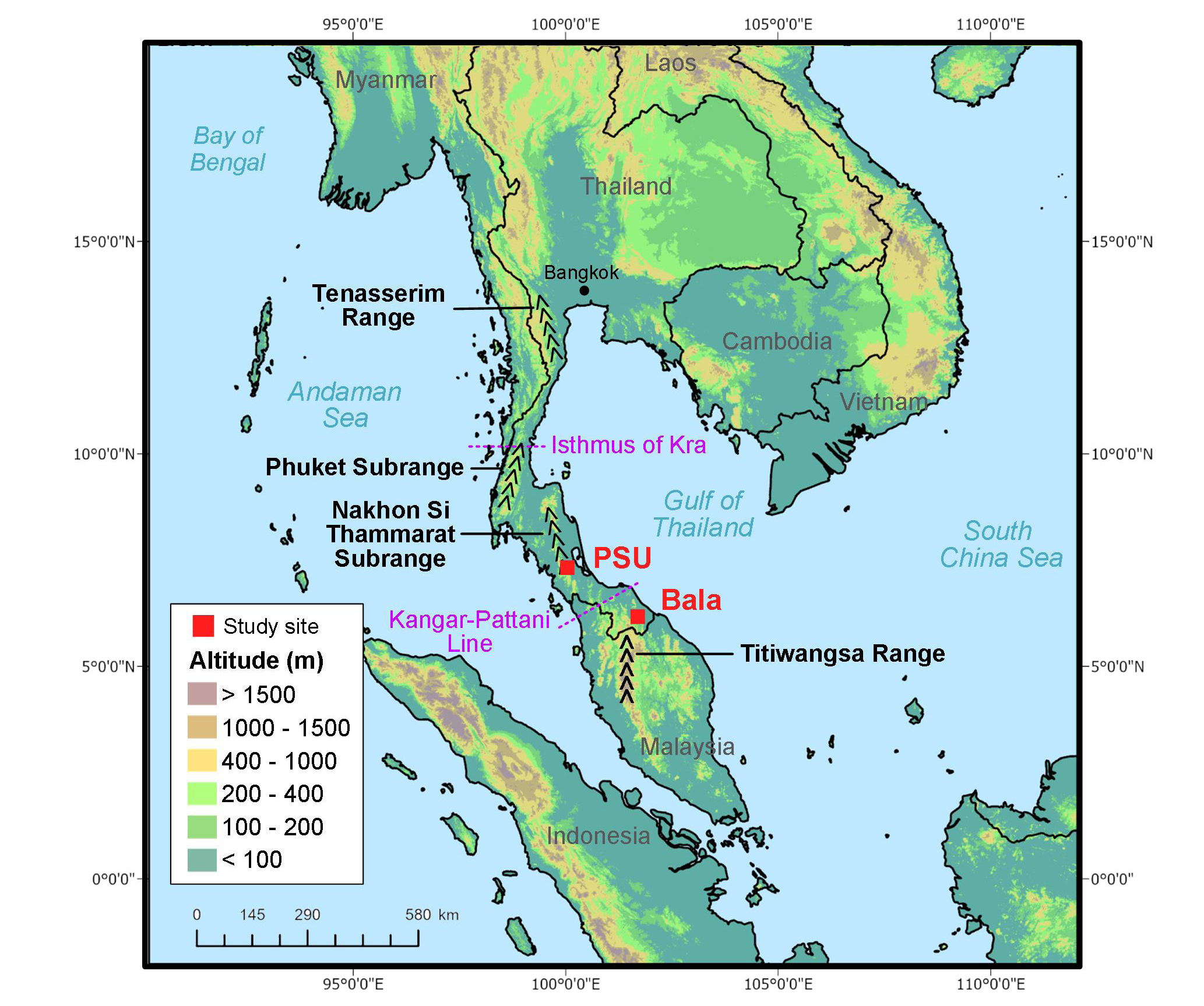
Study sites. Two sites were selected for recording (Figure 1): 1) two recorders were installed at the Prince of Songkla University (PSU) site one recorder was placed at the Hala-Bala Wildlife Sanctuary (Bala) site(Appedix 1: Plate 1 and 2). At PSU, the recorders were set on a forested slope of Kho-Hong Hill adjacent to the eastern side of the campus. Large tracts of rubber plantations used to exist on this slope after the native forest was cleared. In 1976-1980, PSU procured this land for conservation purposes, permitting it to revert to natural habitat through succession (Bumrungsri et al. 2006). Major native trees include Schima wallichii, Castanopsis schefferiana, Memecylon edule, Diospyros frutescenes, Diplospora malaccensis (Bumrungsri et al. 2006); the most common understory species (scrubs and treelets) include Ixora javanicai, Psederanthemum graciliflorum, Mesua kunstleri (Maxwell 2006). One recorder (PSU1) was set in a spot where abandoned rubber trees are still conspicuous but where undergrowth and some native trees have returned. The other recorder (PSU2) was set closer to the ridge where there was no evidence of rubber trees and where much denser undergrowth was observed. At Bala, a single recorder was set in the vicinity of a stream in a mature lowland dipterocarp forest near the research station compound (canopy height ca. 40 to 60 m, composed of Malaysian flora; Kitamura et al. 2011). The lower level of the forest at this site is covered by dense undergrowth. The only light penetrating is through occasional gaps in the canopy. Both sites are of typical tropical climate with alternating wet-dry seasons. Previously recorded data (1981 to 2010) indicate that average monthly rainfall for the month of October (early wet season) was about 2 to 2.5 times that for the month of June (late dry season), 257/100 mm at PSU and 254/123 mm at Bala (Thai Meterological Department, https://www.tmd.go.th/en).
Recording setup. We conducted audio recordings during two periods—June 2023 and October 2023—corresponding roughly to the late dry season and the early wet season in Southern Thailand, respectively. At Bala, we recorded in both seasons but recording files of the dry season were lost due to a malfunction of the recorder. Song Meter Mini Bat 2 AA recorders were used in this study (Wildlife Acoustic, Maryland, MA, USA). Each recorder was mounted on a tree at a height of 1.5 to 1.8 m aboveground and data disks were collected at the end of the month. The recorders’ sampling frequencies were set to 384 kHz capturing a one-minute file every fifteen minutes from evening to dawn (16:00 to 7:00 hr).
Acoustic analysis. Sound files (.wav) were analyzed using Kaleidoscope Lite Analysis software (version 5.6.2; Wildlife Acoustic, Maryland, MA, USA). Each one-minute recording was segmented into twelve 5-second windows for detailed analysis, after which bat calls were manually screened and labeled by one of us (Grace Rui-Tong Yu), after which they were identified to species by a local bat specialist (Pipat Soisok) who has extensive experience capturing and recording bat species in the region. Four characteristic measurements were used for identification: frequency of maximal energy, start frequency, terminal frequency, and call duration. All call files were deposited in the Natural History Museum of Prince Songkla University and are available upon request. We treated windows that contained bat calls involving at least three repetitions as a passing (fly by) event. Since we could not ascertain whether all the calls in one window were produced by one bat or several bats, we quantified them as one passing. Windows with calls that clearly overlapped or contained different signal structures were counted as separate passings. To enhance the detection of legitimate bat calls, the software's signal detection parameters were set as follows: frequency range 12 kHz to 180 kHz, pulse length 1 ms to 80 ms, maximum inter-pulse gap 500 ms, and a minimum of 3 pulses per detection. Files not fulfilling these requirements were labeled as “noise” files. To prevent mislabeling files that might contain legitimate bat calls, we randomly selected files labeled as “noise” for re-screenings, but this reassessment did not retrieve any legitimate bat calls that had been labeled as “noise”. Therefore, all noise files were purged without further analysis. Spectrograms (.png) were made by BatSound (version; Pettersson Elektronik AB, Uppsala, Sweden) with the following settings: sampling rate of 384000, FFT size of 1024, FFT overlap of 0, 16 bits sample, and the Hanning window.
Results
At the PSU sites, a total of 9,744 5-sec recording windows were retrieved (PSU1: 4,704 and PSU2: 5,040, respectively); at PSU1, 364 (7.7 %) of these windows contained bat signals (dry season: 321; wet season: 43); at PSU2, bat signals occurred in 1,280 (25.4 %) of the windows examine (dry season: 572; wet season: 708). At the Bala site, 4,692 recording windows were retrieved in the wet season; 342 (7.3 %) of those time windows had bat signals. Thus, the highest numbers of bat signals were detected at PSU2 during the wet season.
Signals from ten species of bats were recognized (Figure 2 and Table 1), including three unidentified species: four CF (constant frequency) species - Rhinilopus luctus (Rl), R. trifoliatus (Rt), R. acuminatus (Ra), and R. refulgens (Rr), and six FM (frequency modulation) species - Taphozous melanopogan (Tm), Sonotype 1 (S1), Scotophilus kuhlii (Sk), Sonotype 2 (S2), Myotis horsfieldii (Mh), and Kerivoula sp. (K1). Sonotype 1 likely belonged to an unknown species of Myotis and Sonotype 2 to Pipistrellus.
The potential for interspecific interactions could be inferred from the time windows with multiple bat occurrences despite the low number of such time windows: only 81 (4.5 %) out of a total of 1,816 time windows had multiple bat calls. Among those 81 time windows, 47 time windows contained calls of Myotis horsfeildii co-occurring with six other species and 40 time windows contained calls of Scotophilus kuhlii co-occurring with five other species. Given that these two species were the second and third most common species in our study, these results are not surprising. In contrast, however, the most common species Rhinolophus trifoliatus, co-occurred with two other species in just 20 time windows. This, in part, may attest to the distinct foraging strategy of Rhinolophus trifoliatus using CF echolocation, which reduced the probability of their calls being recorded with other species in the same time window. Further testing will be needed to understand the spatial proximity associated with these acoustic co-occurrences.
Eight species and six species were recorded at PSU and Bala, respectively (Table 2). However, taking passing counts of 30 as a cutoff, only three species (R. trifoliatus, M. horsfieldii, and S. kuhlii) at PSU and two species (Sonotype 1 and M. horsfieldii) at Bala were considered common. Four species of Rhinolophus were detected in PSU but none in Bala, whereas Kerivoula, despite small passing counts, was detected only in Bala. Only one species (M. horsfieldii) was common at both PSU and Bala. Finally, hunting buzz calls were mostly detected at Bala (Table 2).
At PSU sites, seasonal variation in the calls recorded was only noted at PSU1, with more passing counts in the dry season than in the wet season (Table 2), primarily for S. kuhlii and M. horsfieldii (ten times or greater difference).
Diel activity patterns, as reflected by passing counts of four common species (R. trifoliatus, S. kuhlii, M. horsfieldii, and Sonotype 2), were also examined (Figure 3). All except one species (R. trifoliatus) began to emerge at 17:00 hr, with R. trifoliatus emerging one hour later at 18:00 hr. At 06:00 hr, no passings were detected for Sonotype 2 while the other three species were still active at 07:00 hr. R. trifoliatus showed the most marked two-peak activity pattern, with a first peak at 21:00 hr and a second at 02:00 hr. More modest two-peak activity patterns were seen in the other three species, with varying peak times (Figure 3).
Discussion
Our trial of deploying automated bat recorders in forests in Southern Thailand was proven efficient in detecting diversity and activity patterns in a short period of recording time. Within one week, we documented 10 species of bats from their acoustic signals, including three unidentified species. Due to the continuous nature of the recordings, we were given a glimpse into diel activity patterns of these species as well, which would have been much more difficult to obtain without substantial effort to capture the bats. Finally, the close co-occurrence of different bat species could be inferred by examining overlapping of acoustic signals of multiple bat species within recording windows.
One drawback in attempting to identify bat species by their acoustic signals in this region is that only a few studies on bat echolocation are available for comparison despite the high level of bat diversity. Even though our identification for bat species based on acoustic signals relied largely upon a residential bat specialist (Pipat Soisok) who is experienced with local bat fauna and their call signals, we obtained some acoustic signals for which positive species identification could not be made. In those cases, species assignments were treated as tentative awaiting further clarification. Furthermore, knowledge on foraging behavior/ecology for some species has cast doubt on species identification. For instance, Scotophilus kuhlii (Zhu et al. 2012) and Myotis horsfieldii (Haslauer 2019) are known to be open-space foragers and yet their calls were common in our recordings despite the fact that our recorders were installed in lower levels within forests. Conversely, the ability to detect behaviors not previously reported could turn out to be an under-appreciated merit of studying bats with automated recorders. Our current understanding of Scotophilus kuhlii is that this species is often associated with humans and congregates in roosting sites on cultivated palm trees (Zhu et al. 2012). At the Bala site, which is deep in a forested area, native palm trees are common and could be the original roosting sites for this species prior to human arrivals.
Three different habitat types were chosen for this study: PSU1, which includes a recently abandoned rubber plantation, PSU2, which is composed of less disturbed forest adjacent to a rubber plantation, and Bala, which is considered a natural forest. Our preliminary results indicate that once rubber plantations are set aside and ecological succession is allowed to proceed, bat species richness approaches that observed in natural forests. However, the composition of such assemblages (Table 2) differs in these two habitats, likely because structures of the forest types remain distinct and thus ecological niches within these habitat types still differ.
Due to its unique geographical position, Peninsular Thailand is a biodiversity hotspot, where Sundaland, Indochinese and Indo-Burmese faunal components converge and interchange (Myers et al. 2010). As a result, numerous national parks (39, including 4 proposed), forest parks (8), non-hunting areas (18), and wildlife sanctuaries (15) have been established in this region. Yet, land use in Southern Thailand is still shifting. Automated recordings have the potential not only to uncover aspects of bat ecology that were rarely known in the past, but also to monitor bat communities across these ever-changing landscapes.
Finally, diel activity patterns extracted from our data are interesting and important in revealing temporal niche separation by sympatric bat species. One example is R. trifoliatus and M. horsfieldii, which are sympatric at the PSU site (Table 2). While activity of R. trifoliatus reached peaks at 21:00 and 2:00 hr, peak times of M. horsfieldii were at 22:00 and 1:00 hr (Figure 3). Both of these species are known to forage in open space so temporal niche segregation could facilitate their co-occurrence. Because bats are the most mobile terrestrial mammals and are sensitive to subtle environmental changes, collecting acoustic signals at various structural positions within a forest to reveal diel activity patterns would provide a valuable means to understand their ecological roles and to monitor environmental changes.
In conclusion, our trials involving automated bat recorders are useful in documenting bat diversity, activity patterns of bats, and the potential for interspecific interactions. Nonetheless, studies of the acoustics of live bats that can be positively identified, preferably from a larger geographical realm, will increase the accuracy of assigning species identification to acoustic signals. Moreover, it is crucial that recorders be set in a wide variety of habitat structures and habitat types. For example, it will be important to position the recorders in a way that captures the vertical stratification within a forest as well as distinct topological features, such as in cleared open passageways or forest streams. Long-term collection and curation of bat calls using automated recorders will prove to be fruitful in expanding our understanding of the biodiversity of this region and the conservation of these changing landscapes.
Acknowledgments
We wish to thank Dr. Ying-Yi Ho of Biodiversity Research Center, Academia Sinica, for his unreserved sharing of analytical skills in acoustics and profound knowledge on bats, and Dr. Chia-Ying Ko of Institute of Fisheries Science, National Taiwan University, for help in making the map. This work is partially supported by the Biodiversity Research Center, Academia Sinica to Mao-Ning Tuanmu.
Epilogue. In Feb 1986, I (Alex Yu) received a letter (done with a typewriter sent by snail mail) from a professor named James Patton, saying that he would accept me as a doctoral student and looked forward to working with me for several years. The English word “several” was just as vague as the city of Berkeley to me at the time. It turns out to be a precious relationship that has lasted for decades. Both Jim and Carol have become the backbone of this relationship. My wife Yulan (we got married in 1987) and later my daughter Grace (born in 2001) joined, too. The education and affection bestowed by Jim and Carol upon us are incredibly profound. We thank them with this little paper that was de facto started by that one-page hand-typed letter that reached me in 1986.
Literature cited
Bumrungsri, S., E. Sripaso-raya, and C. Leelatiwong. 2006. A quantitative analysis of plant community structure in an abandoned rubber plantations on Kho-Hong Hill, southern Thailand. Songklanakarin Journal of Science and Technology 28:479-491.
Dallinger, J. 2011. Oil palm development in Thailand: economic, social and environmental considerations. Pp. 24-51, in Oil Palm Expansion in South East Asia: Trends and implications for local communities and indigenous peoples. RECOFTC, Forest Peoples Programme, Perkumpulan Sawit Watch, Samdhana Institue.
Dejtaradol, A., et al. 2016. Indochinese-Sundaic faunal transition and phylogeographical divides north of the isthmus of Kra in southeast Asian Bulbuls (Aves: Pycnonotidae). Journal of Biogeography 43:471-483.
Denzinger, A., M. Tschapka, and H. U. Schnitzler. 2018. the role of echolocation strategies for niche differentiation in bats. Canadian Journal of Zoology 96:171-181.
Haslauer, R. 2019. Myotis horsfieldii. Pp. 967-968, in Handbook of the Mammals of World - Volume 9: Bats. (Wilson D. E., and R. A. Mittermeier, eds.). Lynx Nature Books, Barcelona, Spain.
Hinckley, A., et al. 2023. evolutionary history and patterns of divergence in three tropical East Asian squirrels across the Isthmus of Kra. Journal of Biogeography 50:1090-102.
Hughes, A. C., et al. 2011. using echolocation calls to identify thai bat species: Vespertilionidae, Emballonuridae, Nycteridae and Megadermatidae. Acta Chiropterologica 13:447-55.
Hughes, J. B., P. D. Round, and D. S. Woodruff. 2003. The Indochinese-Sundaic Faunal Transition at the Isthmus of Kra: An Analysis of Resident Forest Bird Species Distributions. Journal of Biogeography 30:569-80.
Ith, S., et al. 2011. A Taxonomic Review of Rhinolophus Coelophyllus Peters 1867 and R. Shameli Tate 1943 (Chiroptera: Rhinolophidae) in Continental Southeast Asia. Acta Chiropterologica 13:41-59.
Li, F. Y., and S. Q. Li. 2018. Paleocene-Eocene and Plio-pleistocene sea-level changes as "species pumps" in southeast Asia: evidence from Althepus spiders. Molecular Phylogenetics and Evolution 127:545-55.
Karapan, S., A. Wongwai, and P. Soisook. 2023. Cave-dwelling bats of Thailand. wildlife research division, department of national parks, wildlife and plant conservation. Bangkok.
Kitamura, S., et al. 2011. Characteristics of hornbill-dispersed fruits in lowland Dipterocarp forests of southern Thailand. The Raffles Bulletin of Zoology 24:137-147
Lohman, D. J., et al. 2011. Biogeography of the Indo-Australian Archipelago. In annual review of ecology, evolution, and systematics 42:205-26.
Maxwell, J. F. 2006. Vascular flora of Ko Hong Hill, Songkla Province Thailand. Thai Studies in Biodiversity.
Myers, N., et al. 2000. Biodiversity hotspots of conservation priorities. Nature 403:853-858.
Olson, D. M., et al. 2001. Terrestrial ecoregions of the worlds: a new map of life on earth. Bioscience 51:933-38.
Phommexay, P., et al. 2011. The impact of rubber plantations on the diversity and activity of understorey insectivorous bats in southern Thailand. Biodiversity and Conservation 20:1441-1456.
Poyarkov, N. A., et al. 2021. Recent progress in taxonomic studies, biogeographic analysis, and revised checklist of amphibians in Indochina. Russian Journal of Herpetology 28:1-110.
Sethi, S. S., et al. 2020. Characterizing soundscapes across diverse ecosystems using a universal acoustic feature set. Proceedings of the National Academy of Sciences of the United States of America 117:7049-7055.
Soisook, P., M. et al. 2015. Description of a new species of the Rhinolophus Trifoliatus -Group (Chiroptera: Rhinolophidae) from Southeast Asia. Acta Chiropterologica 17:21-36.
Stowell, D., and J. M. Sueur. 2020. Ecoacoustics: acoustic sensing for biodiversity monitoring at scale. Remote sensing in ecology and conservation 6:217-19.
Woodruff, D. S. 2010. Biogeography and conservation in southeast asia: how 2.7 million years of repeated environmental fluctuations affect today's patterns and the future of the remaining refugial-phase biodiversity. Biodiversity and Conservation 19:919-41.
Woodruff, D. S., and L. M. Turner. 2009. The Indochinese-Sundaic zoogeographic transition: a description and analysis of terrestrial mammal species distributions. Journal of Biogeography 36: 803-21.
Yoh, N., et al. 2022. A machine learning framework to classify southeast asian echolocating bats. Ecological Indicators 136:13 108696.
Zhu, G. J., A. Chmura, and L. B. Zhang. 2012. Morphology, echolocation calls and diet of Scotophilus Kuhlii (Chiroptera: Vespertilionidae) on Hainan Island, South China. Acta Chiropterologica 14:175-81.
Associated editors: Marjorie Matocq and Eileen Lacey
Submitted: Sptember 1, 2024; Reviewed: November 2, 2024
Accepted: November 24, 2024; Published on line: January 31, 2025
Appedix 1

Figure 1. Map of Peninsular Thailand showing the locations of automated bat recorders installed. PSU: Prince of Songkla University; Bala: Halabala Wildlife Sanctuary. Isthmus of Kra, Kangar-Pattani Line, and major mountain subranges and ranges are also shown. The altitude data are taken every 7.5-arc-second spatial resolution from the Global Multi-resolution Terrain Elevation Data 2010 (GMTED2010).
Table 1. Measurements for bat calls. fmaxe: frequency of maximal energy; sf: start frequency; tf: terminal frequency; d: call duration.
|
Species |
n |
(fmaxe: kHz) |
(sf: kHz) |
(tf: kHz) |
(d: ms) |
|
Rhinolophus luctus |
4 |
41.3 ± 0.2 |
37.1 ± 0.9 |
38.8 ± 0.6 |
59.3 ± 2.7 |
|
(41.1-41.5) |
(36.4-38.2) |
(38.4-39.7) |
(56.9-63) |
||
|
Rhinolophus trifoliatus |
19 |
52.7 ± 0.8 |
49.1 ± 3.3 |
47.9 ± 4.3 |
46.6 ± 6.4 |
|
(51.3-53.5) |
(43-53.1) |
(41.4-52.6) |
(38-66) |
||
|
Rhinolophus acuminatus |
20 |
89.8 ± 2.7 |
77.9 ± 7.6 |
78.8 ± 7.9 |
51.7 ± 8.7 |
|
(83.4-92.9) |
(68-92.1) |
(65.4-92.1) |
(35-67.5) |
||
|
Rhinolophus refulgens |
5 |
95.3 ± 0.7 |
76.6 ± 3.0 |
81.2 ± 10.8 |
50.2 ± 12.6 |
|
(94.5-96.2) |
(72.6-80.3) |
(71.8-93.3) |
(29-61) |
||
|
Taphozous melanopogan |
3 |
28.6 ± 1.1 |
38.7 ± 4.8 |
24.5 ± 3.0 |
27.7 ± 36.3 |
|
(27.7-29.8) |
(35.1-44.1) |
(21.5-27.5) |
(1-69) |
||
|
Scotophilus kuhlii |
16 |
40.8 ± 2.0 |
51.7 ± 7.0 |
38.7 ± 2.6 |
11.3 ± 4.0 |
|
(35.9-43.1) |
(44-69.2) |
(34.7-42.6) |
(5.1-17) |
||
|
Sonotype 1 |
1 |
40.7 |
56.1 |
30.1 |
6.9 |
|
Mytois horsfieldii |
20 |
63.0 ± 6.4 |
97.6 ± 8.5 |
38.4 ± 3.4 |
5.0 ± 0.7 |
|
(52.6-69.9) |
(80.7-107.7) |
(32.5-47.4) |
(3.8-6.8) |
||
|
Sonotype 2 |
17 |
54.3 ± 3.9 |
75.7 ± 11.8 |
50.6 ± 2.8 |
4.7 ± 1.0 |
|
(48.9-60.1) |
(58.4-92.1) |
(47.5-54.9) |
(2.3-6.9) |
||
|
Kerivoula sp.1 |
11 |
127.2 ± 8.0 |
163.7 ± 2.0 |
93.0 ± 9.7 |
2.7 ± 0.4 |
|
(105.5-133.1) |
(160-166.7) |
(80.8-103.7) |
(1.9-3.1) |
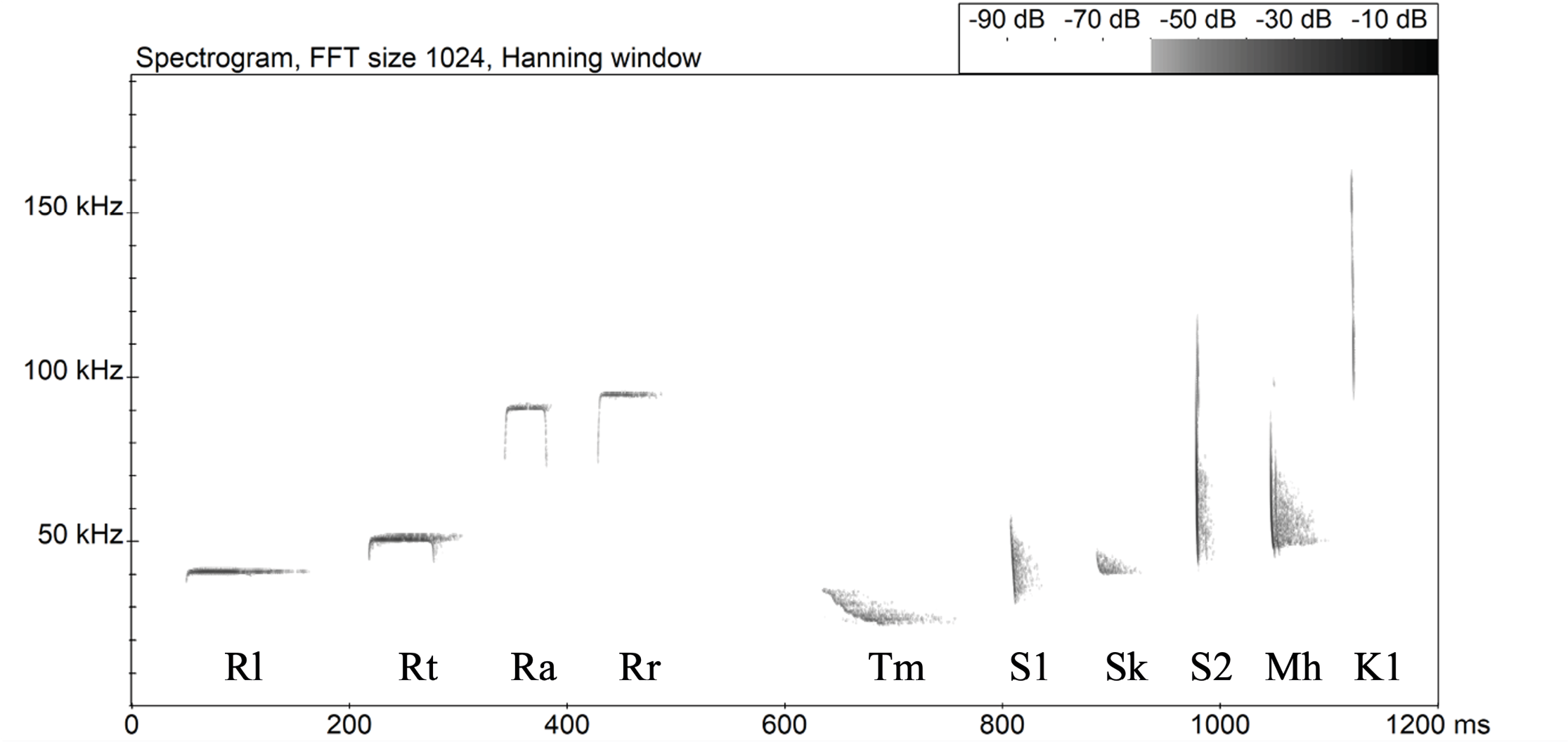
Figure 2. Spectrograms of representative calls of 10 bat species recorded at PSU and Bala. Four CF species: Rhinilopus luctus (Rl), R. trifoliatus (Rt), R. acuminatus (Ra), and R. refulgens (Rr). Six FM species: Taphozous melanopogan (Tm), Sonotype 1 (S1), Scotophilus kuhlii (Sk), Sonotype 2 (S2), Myotis horsfieldii (Mh), and Kerivoula sp. (K1).
Table 2. Passing counts of bats as detected by acoustic signals in the 5-second time windows.
|
Location-season/Species |
PSU1_dry |
PSU2_dry |
PSU1_wet |
PSU2_wet |
BALA_wet |
Sum |
|
Rhinolophus luctus |
12 |
13 |
0 |
26 |
2 |
53 |
|
Rhinolophus trifoliatus |
1 |
344 |
19 |
553 |
0 |
917 |
|
Rhinolophus acuminatus |
22 |
2 |
0 |
3 |
0 |
27 |
|
Rhinolophus refulgens |
4 |
0 |
1 |
0 |
0 |
5 |
|
Taphozous melanopogan |
5 |
1 |
0 |
3 |
0 |
9 |
|
Scotophilus kuhlii |
177 |
61 |
11 |
57 |
29 |
335 |
|
Sonotype 1 |
0 |
0 |
0 |
0 |
1 |
1 |
|
Mytois horsfieldii |
100 |
148 |
10 |
59 |
103 |
420 |
|
Sonotype 2 |
0 |
3 |
2 |
7 |
196 |
208 |
|
Kerivoula sp.1 |
0 |
0 |
0 |
0 |
11 |
11 |
|
|
||||||
|
Sum |
321 |
572 |
43 |
708 |
342 |
1986 |
|
Feeding buzz |
0 |
1 |
0 |
0 |
50 |
51 |
|
Total species |
7 |
7 |
5 |
7 |
6 |
|
|
|
|
8 |
|
|
6 |
|
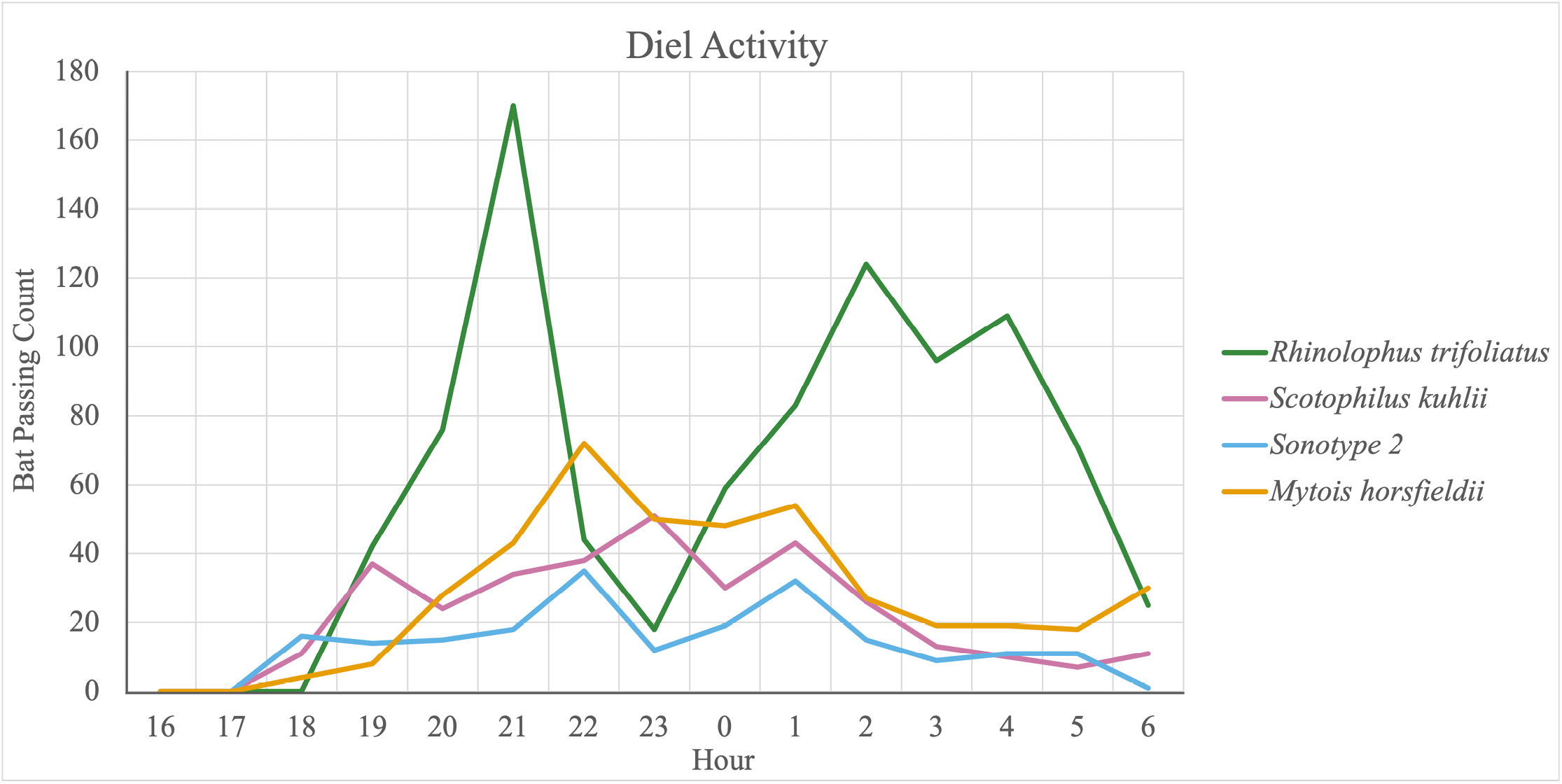
Figure 3. Diel activity patterns of four common species of bats in the southern region of Peninsular Thailand.

Plate 1: Forested slope adjacent to the campus of Prince Songkla University in Peninsular Thailand. This site is an abandoned rubber plantation.

Plate 2. Natural tropical rainforest in Hala-Bala Wildlife Sanctuary. Dipterocarps are dominant tree species here.